Originally posted on DRUG REGULATORY AFFAIRS INTERNATIONAL:

All participants of the GMP training course “GMP-compliant Product Transfer” will receive a special version of the Guideline Manager CD including documents and templates useable for site change projects. Read more.
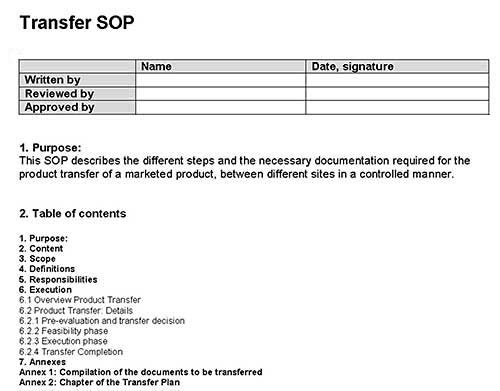
According to the European GMP-Rules, written procedures for tranfser activities and their documentation are required. For example, a Transfer SOP, a transfer plan and a report are now mandatory and will be checked during inspections.
As a participant of the GMP education course “GMP-compliant Product Transfer” in Prague, from 20-22 October 2015 you will receive a special version of the Guideline Manager CD with a special section concerning product transfers. This section contains, amongst others, a Transfer SOP and a template for a Transfer Plan. Both documents are in Word format and can immediately be used after adoption to your own situation.
Regulatory Guidance Documents like the WHO guideline on transfer of technology in pharmaceutical manufacturing and the…
View original 36 more words
Filed under: Uncategorized