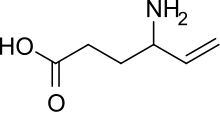
Vigabatrin
CAS: 60643-86-9
- Molecular FormulaC6H11NO2
- Average mass129.157 Da
Infantile spasms, Anticonvulsant, Antiepileptic
orphan drug designation
GVG
M071754
MDL-71754
ORP-001
RMI-71754
RMI-71890 ((+)-enantiomer)
Vigabatrin, brand name Sabril, is an antiepileptic drug that inhibits the breakdown of γ-aminobutyric acid (GABA) by acting as a suicide inhibitor of the enzyme GABA transaminase (GABA-T). It is also known as γ-vinyl-GABA, and is a structural analogue of GABA, but does not bind to GABA receptors.[1]
Medical uses
Epilepsy
In Canada, vigabatrin is approved for use as an adjunctive treatment (with other drugs) in treatment resistant epilepsy, complex partial seizures, secondary generalized seizures, and for monotherapy use in infantile spasms in West syndrome.[1]
As of 2003, vigabatrin is approved in Mexico for the treatment of epilepsy that is not satisfactorily controlled by conventional therapy (adjunctive or monotherapy) or in recently diagnosed patients who have not tried other agents (monotherapy).[2]
Vigabatrin is also indicated for monotherapy use in secondarily generalized tonic-clonic seizures, partial seizures, and in infantile spasms due to West syndrome.[2]
On August 21, 2009, Lundbeck announced that the U.S. Food and Drug Administration had granted two New Drug Application approvals for vigabatrin. The drug is indicated as monotherapy for pediatric patients one month to two years of age with infantile spasms for whom the potential benefits outweigh the potential risk of vision loss, and as adjunctive (add-on) therapy for adult patients with refractory complex partial seizures (CPS) who have inadequately responded to several alternative treatments and for whom the potential benefits outweigh the risk of vision loss.
In 1994, Feucht and Brantner-Inthaler reported that vigabatrin reduced seizures by 50-100% in 85% of children with Lennox-Gastaut syndrome who had poor results with sodium valproate.[3]
Others
Vigabatrin reduced cholecystokinin tetrapeptide-induced symptoms of panic disorder, in addition to elevated cortisol and ACTH levels, in healthy volunteers.[4]
Vigabatrin is also used to treat seizures in succinic semialdehyde dehydrogenase deficiency (SSADHD), which is an inborn GABA metabolism defect that causes intellectual disability, hypotonia, seizures, speech disturbance, and ataxia through the accumulation of γ-Hydroxybutyric acid (GHB). Vigabatrin helps lower GHB levels through GABA transaminase inhibition. However, this is in the brain only; it has no effect on peripheral GABA transaminase, so the GHB keeps building up and eventually reaches the brain.[5]
Adverse effects
Central nervous system
Sleepiness (12.5%), headache (3.8%), dizziness (3.8%), nervousness (2.7%), depression (2.5%), memory disturbances (2.3%), diplopia (2.2%), aggression (2.0%), ataxia (1.9%), vertigo (1.9%), hyperactivity (1.8%), vision loss (1.6%) (See below), confusion(1.4%), insomnia (1.3%), impaired concentration (1.2%), personality issues (1.1%).[1] Out of 299 children, 33 (11%) became hyperactive.[1]
Some patients develop psychosis during the course of vigabatrin therapy,[6] which is more common in adults than in children.[7] This can happen even in patients with no prior history of psychosis.[8] Other rare CNS side effects include anxiety, emotional lability, irritability, tremor, abnormal gait, and speech disorder.[1]
Gastrointestinal
Abdominal pain (1.6%), constipation (1.4%), vomiting (1.4%), and nausea (1.4%). Dyspepsia and increased appetite occurred in less than 1% of subjects in clinical trials.[1]
Body as a whole
Fatigue (9.2%), weight gain (5.0%), asthenia (1.1%).[1]
Teratogenicity
A teratology study conducted in rabbits found that a dose of 150 mg/kg/day caused cleft palate in 2% of pups and a dose of 200 mg/kg/day caused it in 9%.[1] This may be due to a decrease in methionine levels, according to a study published in March 2001.[9] In 2005, a study conducted at the University of Catania was published stating that rats whose mothers had consumed 250–1000 mg/kg/day had poorer performance in the water maze and open-field tasks, rats in the 750-mg group were underweight at birth and did not catch up to the control group, and rats in the 1000 mg group did not survive pregnancy.[10]
There is no controlled teratology data in humans to date.
Sensory
In 2003, vigabatrin was shown by Frisén and Malmgren to cause irreversible diffuse atrophy of the retinal nerve fiber layer in a retrospective study of 25 patients.[11] This has the most effect on the outer area (as opposed to the macular, or central area) of the retina.[12] Visual field defects had been reported as early as 1997 by Tom Eke and others, in the UK. Some authors, including Comaish et al. believe that visual field loss and electrophysiological changes may be demonstrable in up to 50% of Vigabatrin users.
The retinal toxicity of vigabatrin can be attributed to a taurine depletion.[13]
Interactions
A study published in 2002 found that vigabatrin causes a statistically significant increase in plasma clearance of carbamazepine.[14]
In 1984, Drs Rimmer and Richens at the University of Wales reported that administering vigabatrin with phenytoin lowered the serum phenytoin concentration in patients with treatment-resistant epilepsy.[15] Five years later, the same two scientists reported a fall in concentration of phenytoin of 23% within five weeks in a paper describing their failed attempt at elucidating the mechanism behind this interaction.[16]
Pharmacology
Vigabatrin is an irreversible mechanism-based inhibitor of gamma-aminobutyric acid aminotransferase (GABA-AT), the enzyme responsible for the catabolism of GABA, which increases the level of GABA in the brain.[1][17] Vigabatrin is a racemic compound, and its [S]-enantiomer is pharmacologically active.[18],[19]
Crystal Structure (pdb:1OHW) showing vigabatrin binding to specific residues in the active site of GABA-AT, based off experiments by Storici et al.[20]
Pharmacokinetics
With most drugs, elimination half-life is a useful predictor of dosing schedules and the time needed to reach steady state concentrations. In the case of vigabatrin, however, it has been found that the half-life of biologic activity is far longer than the elimination half-life.[21]
For vigabatrin, there is no range of target concentrations because researchers found no difference between the serum concentration levels of responders and those of non-responders.[22] Instead, the duration of action is believed to be more a function of the GABA-T resynthesis rate; levels of GABA-T do not usually return to their normal state until six days after stopping the medication.[19]
History
Vigabatrin was developed in the 1980s with the specific goal of increasing GABA concentrations in the brain in order to stop an epileptic seizure. To do this, the drug was designed to irreversibly inhibit the GABA transaminase, which degrades the GABA substrate. Although the drug was approved for treatment in the United Kingdom in 1989, the authorized use of Vigabatrin by US Food and Drug Administration was delayed twice in the United States before 2009. It was delayed in 1983 because animal trials produced intramyelinic edema, however, the effects were not apparent in human trials so the drug design continued. In 1997, the trials were temporarily suspended because it was linked to peripheral visual field defects in humans.[23]
Society and culture
Brand Names
Vigabatrin is sold as Sabril in Canada,[24] Mexico,[2] and the United Kingdom.[25] The brand name in Denmark is Sabrilex. Sabril was approved in the United States on August 21, 2009 and is currently marketed in the U.S. by Lundbeck Inc., which acquired Ovation Pharmaceuticals, the U.S. sponsor in March 2009.
Synthesis
http://www.drugfuture.com/synth/syndata.aspx?ID=90252

This compound can be prepared in two different ways: 1) The reaction of 1,4-dichloro-2-butene (I) with diethyl malonate (II) by means of sodium ethoxide in refluxing ethanol gives 1,1-bis(ethoxycarbonyl)-2-vinylcyclopropane (III), which by reaction with ammonia gas in DMF at 120 C is converted into 3-carboxamido-5-vinyl-2-pyrrolidone (IV). Finally, this compound is treated with concentrated HCl in refluxing acetic acid. 2) The treatment of (IV) with sodium ethoxide in refluxing ethanol gives 3-carboxy-5-vinyl-2-pyrrolidone (V), which is decarboxylated by treatment with refluxing acetic acid to afford 5-vinyl-2-pyrrolidone (VI). The bromination of (VI) with Br2 in CCl4 yields 5-(1,2-dibromoethyl)-2-pyrrolidone (VII), which by treatment with Na in liquid NH3 in a pressure vessel at 25 C is converted into 4-aminohex-5-inoic acid (VIII). Finally, this compound is partially reduced with H2 over a suitable catalyst.

The synthesis of [14C]-labeled vigabatrin has been described: The reduction by known methods of pyroglutamic acid (I) to the alcohol (II) and its acylation with p-toluenesulfonyl chloride gives 5-(tosyloxymethyl)pyrrolidin-2-one (III), which by reaction with [14C]-labeled sodium cyanide in hot DMF yields 5-([14C]-cyanomethyl)pyrrolidin-2-one (IV). The reduction of (VI) with H2 over Pd/Al2O3 and treatment with dimethylamine affords 5-[2-(dimethylamino)ethyl]pyrrolidin-2-one (VI), which is oxidized with H2O2 in water to the N-oxide (VI). The treatment of (VI) with K2CO3 in refluxing xylene affords 5-([14C]-vinyl)pyrrolidin-2-one (VII), which is finally submitted to ring opening with hot 5 M aqueous HCl, followed by neutralization with triethylamine.

An efficient new synthesis for [14C]-labeled vigabatrin has been described: The reaction of triphenylphosphine (I) with [14C]-labeled methyl iodide (II) in benzene gives the corresponding phosphonium salt (III), which is submitted to a Wittig condensation with 1-(1-butenyl)-5-oxopiperidin-2-carbaldehyde (IV) to afford the vinylpyrrolidone (V). Finally, this compound is hydrolyzed with 6N HCl at 95 C.
 The enantiocontrolled addition of phthalimide (I) to 1,3-butadiene monoepoxide (II) with a chiral palladium catalyst and Na2CO3 in dichloromethane gives N-(2-hydroxy-1(S)-vinylethyl)phthalimide (III), which is treated with triflic anhydride and TEA in dichloromethane to yield the triflate (IV). The condensation of (IV) with dimethyl malonate (V) by means of NaH in THF affords the alkylated malonate (VI), which is finally decarboxylated and deprotected by a treatment with aqueous refluxing HCl. Note that the synthesis of the biologically active (S)-enantiomer simply requires a change in the chirality of the Pd catalyst used in the first step of the synthesis.
The enantiocontrolled addition of phthalimide (I) to 1,3-butadiene monoepoxide (II) with a chiral palladium catalyst and Na2CO3 in dichloromethane gives N-(2-hydroxy-1(S)-vinylethyl)phthalimide (III), which is treated with triflic anhydride and TEA in dichloromethane to yield the triflate (IV). The condensation of (IV) with dimethyl malonate (V) by means of NaH in THF affords the alkylated malonate (VI), which is finally decarboxylated and deprotected by a treatment with aqueous refluxing HCl. Note that the synthesis of the biologically active (S)-enantiomer simply requires a change in the chirality of the Pd catalyst used in the first step of the synthesis.

The reaction of 3-aminotetrahydrofuran-2-one (I) with benzyloxycarbonyl chloride (II) and TEA in chloroform gives the carbamate (III), which is reduced to the lactol (IV) by means of DIBAL in toluene. It has been observed that lactol (IV) is in equilibrium with its tautomeric open chain aldehydic form.(V). The reaction of (IV)??(V) with phosphonium bromide (VI) by means of Bu-Li in THF yields 3-amino-4-penten-1-ol (VII), which is reprotected with benzyloxycarbonyl chloride (II) and TEA to afford the carbamate (VIII). The reaction of (VIII) with CBr4 and PPh3 in dichloromethane provides the pentenyl bromide (IX), which is treated with LiCN in THF to give 4-(benzyloxycarbonylamino)-5-hexenenitrile (X). Finally this compound is hydrolyzed with conc. HCl to yield the target 4-amino-5-hexenoic acid.
References
- ^ Jump up to:a b c d e f g h i Long, Phillip W. “Vigabatrin.” Archived April 23, 2006, at the Wayback Machine. Internet Mental Health. 1995–2003.
- ^ Jump up to:a b c DEF Mexico: Sabril Archived September 14, 2005, at the Wayback Machine. Diccionario de Especialdades Farmaceuticas. Edicion 49, 2003.
- Jump up^ Feucht M, Brantner-Inthaler S (1994). “Gamma-vinyl-GABA (vigabatrin) in the therapy of Lennox-Gastaut syndrome: an open study” (PDF). Epilepsia. 35 (5): 993–8. doi:10.1111/j.1528-1157.1994.tb02544.x. PMID 7925171. Retrieved 2006-05-25.
- Jump up^ Zwanzger P, Baghai TC, Schuele C, Strohle A, Padberg F, Kathmann N, Schwarz M, Moller HJ, Rupprecht R (2001). “Vigabatrin decreases cholecystokinin-tetrapeptide (CCK-4) induced panic in healthy volunteers”. Neuropsychopharmacology. 25 (5): 699–703. doi:10.1016/S0893-133X(01)00266-4. PMID 11682253.
- Jump up^ Pearl, Phillip L; Robbins, Emily; Capp, Philip K; Gasior, Maciej; Gibson, K Michael (May 5, 2004). “Succinic Semialdehyde Dehydrogenase Deficiency”. GeneReviews. Seattle, Washington: University of Washington. Retrieved September 6, 2010.
- Jump up^ Sander JW, Hart YM (1990). “Vigabatrin and behaviour disturbance”. Lancet. 335 (8680): 57. doi:10.1016/0140-6736(90)90190-G. PMID 1967367.
- Jump up^ Chiaretti A, Castorina M, Tortorolo L, Piastra M, Polidori G (1994). “[Acute psychosis and vigabatrin in childhood]”. La Pediatria Medica e Chirurgica : Medical and surgical pediatrics. 16 (5): 489–90. [Article in Italian] PMID 7885961
- Jump up^ Sander JW, Hart YM, Trimble MR, Shorvon SD (1991). “Vigabatrin and psychosis”. Journal of Neurology, Neurosurgery, and Psychiatry. 54 (5): 435–9. doi:10.1136/jnnp.54.5.435. PMC 488544
![Freely accessible Freely accessible]() . PMID 1865207.
. PMID 1865207. - Jump up^ Abdulrazzaq YM, Padmanabhan R, Bastaki SM, Ibrahim A, Bener A (2001). “Placental transfer of vigabatrin (gamma-vinyl GABA) and its effect on concentration of amino acids in the embryo of TO mice”. Teratology. 63 (3): 127–33. doi:10.1002/tera.1023. PMID 11283969.
- Jump up^ Lombardo SA, Leanza G, Meli C, Lombardo ME, Mazzone L, Vincenti I, Cioni M (2005). “Maternal exposure to the antiepileptic drug vigabatrin affects postnatal development in the rat”. Neurological Sciences. 26 (2): 89–94. doi:10.1007/s10072-005-0441-6. PMID 15995825.
- Jump up^ Frisén L, Malmgren K (2003). “Characterization of vigabatrin-associated optic atrophy”. Acta Ophthalmologica Scandinavica. 81 (5): 466–73. doi:10.1034/j.1600-0420.2003.00125.x. PMID 14510793.
- Jump up^ Buncic JR, Westall CA, Panton CM, Munn JR, MacKeen LD, Logan WJ (2004). “Characteristic retinal atrophy with secondary “inverse” optic atrophy identifies vigabatrin toxicity in children”. Ophthalmology. 111 (10): 1935–42. doi:10.1016/j.ophtha.2004.03.036. PMC 3880364
![Freely accessible Freely accessible]() . PMID 15465561.
. PMID 15465561. - Jump up^ Gaucher D; Arnault E; Husson Z; et al. (November 2012). “Taurine deficiency damages retinal neurones: cone photoreceptors and retinal ganglion cells”. Amino Acids. 43 (5): 1979–1993. doi:10.1007/s00726-012-1273-3. PMC 3472058
![Freely accessible Freely accessible]() . PMID 22476345.
. PMID 22476345. - Jump up^ Sanchez-Alcaraz, Agustín; Quintana MB; Lopez E; Rodriguez I; Llopis P (2002). “Effect of vigabatrin on the pharmacokinetics of carbamazepine”. Journal of Clinical Pharmacology and Therapeutics. 27 (6): 427–30. doi:10.1046/j.1365-2710.2002.00441.x. PMID 12472982.
- Jump up^ Rimmer EM, Richens A (1984). “Double-blind study of gamma-vinyl GABA in patients with refractory epilepsy”. Lancet. 1 (8370): 189–90. doi:10.1016/S0140-6736(84)92112-3. PMID 6141335.
- Jump up^ Rimmer EM, Richens A (1989). “Interaction between vigabatrin and phenytoin”. British Journal of Clinical Pharmacology. 27 (Suppl 1): 27S–33S. doi:10.1111/j.1365-2125.1989.tb03458.x. PMC 1379676
![Freely accessible Freely accessible]() . PMID 2757906.
. PMID 2757906. - Jump up^ Rogawski MA, Löscher W (2004). “The neurobiology of antiepileptic drugs”. Nat Rev Neurosci. 5 (7): 553–564. doi:10.1038/nrn1430. PMID 15208697.
- Jump up^ Sheean, G.; Schramm T; Anderson DS; Eadie MJ. (1992). “Vigabatrin–plasma enantiomer concentrations and clinical effects”. Clinical and Experimental Neurology. 29: 107–16. PMID 1343855.
- ^ Jump up to:a b Gram L, Larsson OM, Johnsen A, Schousboe A (1989). “Experimental studies of the influence of vigabatrin on the GABA system”. British Journal of Clinical Pharmacology. 27(Suppl 1): 13S–17S. doi:10.1111/j.1365-2125.1989.tb03455.x. PMC 1379673
![Freely accessible Freely accessible]() . PMID 2757904.
. PMID 2757904. - Jump up^ Storici Paola; De Biase D; Bossa F; Bruno S; Mozzarelli A; Peneff C; Silverman R; Schirmer T. (2003). “Structures of γ-Aminobutyric Acid (GABA) Aminotransferase, a Pyridoxal 5′-Phosphate, and [2Fe-2S] Cluster-containing Enzyme, Complexed with γ-Ethynyl-GABA and with the Antiepilepsy Drug Vigabatrin”. The Journal of Biochemistry. 279(1): 363–73. doi:10.1074/jbc.M305884200. PMID 14534310.
- Jump up^ Browne TR (1998). “Pharmacokinetics of antiepileptic drugs”. Neurology. 51 (5 suppl 4): S2–7. doi:10.1212/wnl.51.5_suppl_4.s2. PMID 9818917.
- Jump up^ Lindberger M, Luhr O, Johannessen SI, Larsson S, Tomson T (2003). “Serum concentrations and effects of gabapentin and vigabatrin: observations from a dose titration study”. Therapeutic Drug Monitoring. 25 (4): 457–62. doi:10.1097/00007691-200308000-00007. PMID 12883229.
- Jump up^ Ben-Menachem E. (2011). “Mechanism of Action of vigabatrin: correcting misperceptions”. Acta Neurologica Scandinavica. 124: 5. doi:10.1111/j.1600-0404.2011.01596.x.
- Jump up^ drugs.com Vigabatrin Drug Information
- Jump up^ Treatments for Epilepsy – Vigabatrin Norfolk and Waveney Mental Health Partnership NHS Trust
///////////Vigabatrin, ビガバトリン , MDL-71754; RMI-71754, orphan drug designation
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Sabril |
| Synonyms | γ-Vinyl-GABA |
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a610016 |
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 80–90% |
| Protein binding | 0% |
| Metabolism | not metabolized |
| Biological half-life | 5–8 hours in young adults, 12–13 hours in the elderly. |
| Excretion | Renal |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.165.122  |
| Chemical and physical data | |
| Formula | C6H11NO2 |
| Molar mass | 129.157 g/mol |
| 3D model (JSmol) | |
| Melting point | 171 to 177 °C (340 to 351 °F) |



