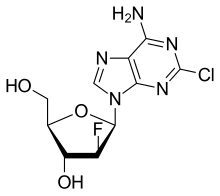
Clofarabine
- Molecular FormulaC10H11ClFN5O3
- Average mass303.677 Da

| CENTRAL DRUGS STANDARD CONTROL ORGANIZATION |
| DIRECTOR GENERAL OF HEALTH SERVICES, |
| MINISTRY OF HEALTH AND FAMILY WELFARE, |
| GOVERNMENT OF INDIA |
approved
|
Clofarabine Bulk & Injection 20 mg/20ml vial
|
For the treatment of patients 1 to 21 years old with relapsed or refractory acute lymphoblastic leukemia after at least two prior regimens. This indication is based upon response rate
|
16.01.2018 |
Clofarabine is a purine nucleoside antimetabolite marketed in the US and Canada as Clolar. In Europe and Australia/New Zealand the product is marketed under the name Evoltra. It is FDA-approved for treating relapsed or refractory acute lymphoblastic leukaemia(ALL) in children after at least two other types of treatment have failed. It is not known if it extends life expectancy. Some investigations of effectiveness in cases of acute myeloid leukaemia (AML) and juvenile myelomonocytic leukaemia (JMML) have been carried out. Ongoing trials are assessing its efficacy, if any, for managing other cancers.
Clofarabine is a purine nucleoside antimetabolite that is being studied in the treatment of cancer. It is marketed in the U.S. and Canada as Clolar. In Europe and Australia/New Zealand the product is marketed under the name Evoltra.
Clofarabine is used in paediatrics to treat a type of leukaemia called relapsed or refractory acute lymphoblastic leukaemia (ALL), only after at least two other types of treatment have failed. It is not known if the drug extends life expectancy. Some investigations of effectiveness in cases of acute myeloid leukaemia (AML) and juvenile myelomonocytic leukaemia (JMML) have been carried out.
For the treatment of pediatric patients 1 to 21 years old with relapsed or refractory acute lymphocytic (lymphoblastic) leukemia after at least two prior regimens. It is designated as an orphan drug by the FDA for this use.
Approval
Clolar was Food and Drug Administration (FDA) approved 28 December 2004. (Under accelerated approval regulations requiring further clinical studies.)

Side effects
- Tumor lysis syndrome (TLS). Clofarabine quickly kills leukaemia cells in the blood. The body may react to this. Signs include hyperkalemia, hyperuricemia, and hyperphosphatemia. TLS is very serious and can lead to death if it is not treated right away.
- Systemic inflammatory response syndrome (SIRS): symptoms include fast breathing, fast heartbeat, low blood pressure, and fluid in the lungs.
- Bone marrow problems (suppression). Clofarabine can stop the bone marrow from making enough red blood cells, white blood cells, and platelets. Serious side effects that can happen because of bone marrow suppression include severe infection (sepsis), bleeding, and anemia.
- Effects on pregnancy and breastfeeding. Girls and women should not become pregnant or breastfeed during treatment which may harm the baby.
- Dehydration and low blood pressure. Clofarabine can cause vomiting and diarrhea which may lead to low body fluid (dehydration). Signs and symptoms of dehydration include dizziness, lightheadedness, fainting spells, or decreased urination.
- Other side effects. The most common side effects are stomach problems (including vomiting, diarrhea, and nausea), and effects on blood cells (including low red blood cells count, low white blood cell count, low platelet count, fever, and infection. Clofarabine can also cause tachycardia and can affect the liver and kidneys.
Contraindications
- pregnancy or planned pregnancy
- breast-feeding
- liver problems
- kidney problems
Drug interactions
- nephrotoxic drugs
- hepatotoxic drugs
Delivery
- By intravenous infusion.
- Dosage is a 2-hour infusion (52 mg/m²) every day for five days. The cycle is repeated every 2 to 6 weeks.
- Regular blood tests to monitor his or her blood cells, kidney function, and liver function.
Biology
Clofarabine is a second-generation purine nucleoside analog designed to overcome biological limitations observed with ara-A and fludarabine. A 2´(S)-fluorine in clofarabine significantly increased the stability of the glycosidic bond in acidic solution and toward phosphorolytic cleavage as compared to fludarabine.[1] A chlorine substitution at the 2-position of the adenine base avoids production of a 2-fluoroadenine analog, a precursor to the toxic 2-fluoro-adenosine-5´-triphosphate and prevents deamination of the base as compared to ara-A.[2]
Clofarabine can be administered intravenously or given orally. Clofarabine enters cells via hENT1, hENT2, and hCNT2, where upon it is phosphorylated by deoxycytidine kinase to generate clofarabine-5´-monophosphate. The rate-limiting step in clofarabine metabolism is clofarabine-5´-diphosphosphate. Clofarabine-5´-triphosphate is the active-metabolite, and it inhibits ribonucleotide reductase, resulting in a decrease cellular dNTP concentrations, which promotes greater incorporation of clofarabine-5´-triphosphate during DNA synthesis. Embedded clofarabine-5´-monophosphate in the DNA promotes polymerase arrest at the replication fork, triggering DNA repair mechanisms that without repair lead to DNA strand breaks in vitro and cytochrome c-mediated apoptosis in vitro. Studies using cell lines have shown that clofarabine-5´-triphosphate can also be incorporated into RNA.[3]
Mechanisms of resistance and turnover have been reported. Clofarabine-resistance arises from decreased deoxycytidine kinase activity in vitro.[4] ABC transporter ABCG2 promotes export of clofarabine-5´-monophosphate and thus limits the cytotoxic effects of this analog in vivo.[5] Biochemically, clofarabine-5’-triphosphate was shown to be substrate for SAMHD1, thus potentially limiting the amount of active compound in cells.[6]

Synthesis
Production of Clofarabine
The reaction flask was added 2-chloro-9-(2-deoxy-2-fluoro-3,5-di-O-benzoyl-beta-D arabinose yl) adenine 1.5g (3mmol) and methanol 40ml,mixed with stirring. Then it was added sodium methoxide, 0.05g (content> 50%), the reaction was stirred for 40min. Then the mixture was cooled to room temperature, adjusted to pH 7 with acetic acid, filtered, and the filter cake was washed with an ice-methanol 10ml, added to the methanol 40ml, and heated to 63 °C, and then cooled to -10 o C. Still 1h, filtered, and the filter cake was washed with an ice-methanol 10ml, drained, dried under reduced pressure to give an off-white powdery solid clofarabine 0.48g. The yield is 54%.

CLIP

http://pubs.rsc.org/en/content/articlehtml/2017/ra/c6ra27790j
CLIP

SYN 1
JP 1993502014; US 5034518; WO 9014352

Reaction of 1,2:5,6-di-O-isopropylidene-3-O-tosyl-a-D-allofuranose (I) with KF in acetamide at 210 oC gives 3-deoxy-3-fluoro-1,2:5,6-di-O-isopropylidene-a-D-glucofuranose (II), which is treated with a 1:1 mixture of metha-nol and 0.7% aqueous H2SO4 to yield 3-deoxy-3-fluoro-1,2-isopropylidene-a-D-glucofuranose (III). Selective acylation of the sugar (III) with benzoyl chloride in pyridine affords the 6-O-benzoyl derivative (IV), which is treated with Amberlite IR-100 (H+) ion-exchange resin in hot dioxane to provide 6-O-benzoyl-3-deoxy-3-fluoro-D-glucofuranose (V). The oxidative cleavage of glucofuranose (V) by means of KIO4 in water results in rearrangement to give 5-O-benzoyl-2-deoxy-2-fluoro-3-O-formyl-D- arabinofuranose (VI), which is deformylated by means of NaOMe in methanol to provide 5-O-benzoyl-2-deoxy-2-fluoro-D-arabinofuranose (VII). Acylation of the arabinofuranose (VII) with acetic anhydride in pyridine affords the 1,3-di-O-acetyl derivative (VIII), which is treated with HBr in AcOH/CH2Cl2 to yield 3-O-acetyl-5-O-benzoyl-2-deoxy-2-fluoro-D-arabinofuranosyl bromide (IX). Condensation of compound (IX) with 2-chloroadenine (X) by means of potassium tert-butoxide in different solvents gives the acylated 2-chloroadenosine derivative (XI), which is finally deacylated by means of NaOMe in methanol
Carbohydr Res 1975,42(2),233
Drugs Fut 2004,29(2),112
| J Med Chem 1992,35(2),397 |
US 2003114663; WO 0311877
CA 2400470; EP 1261350; WO 0160383
References
- Jump up^ Parker WB, Allan PW, Hassan AE, Secrist JA 3rd, Sorscher EJ, Waud WR (Jan 2003). “Antitumor activity of 2-fluoror-2’deoxyadenosine against tumors that express Escherichia coli purine nucleoside phosphorylase”. Cancer Gene Ther. 10 (1): 23–29. doi:10.1038/sj.cgt.7700520. PMID 12489025.
- Jump up^ Bonate PL, Arthaud L, Cantrell WR Jr, Stephenson K, Secrist JA 3rd, Weitman S (Feb 2014). “Discovery and development of clofarabine: a nucleoside analogue for treating cancer”. nat Rev Drug Discov. 5 (10): 855–63. doi:10.1038/nrd2055. PMID 17016426.
- Jump up^ Shelton J, Lu X, Hollenbaugh JA, Cho JH, Amblard F, Schinazi RF (Dec 2016). “Metabolism, Biochemical Actions, and Chemical Synthesis of Anticancer Nucleosides, Nucleotides, and Base Analogs”. Chem Rev. 116 (23): 14379–14455. doi:10.1021/acs.chemrev.6b00209. PMID 27960273.
- Jump up^ Lotfi K, Månsson E, Spasokoukotskaja T, Pettersson B, Liliemark J, Peterson C, Eriksson S, Albertioni F (1999). “Biochemical pharmacology and resistance to 2-chloro-2′-arabino-fluoro-2’deoxyadenosine, a novel analogue of cladribine in human leukemic cells”. Clin Cancer Res. 5 (9): 2438–44. PMID 10499616.
- Jump up^ Nagai S, Takenaka K, Nachagari D, Rose C, Domoney K, Sun D, Sparreboom A, Schuetz JD (Mar 2011). “Deoxycytidine kinase modulates the impact of the ABC transporter ABCG2 on clofarabine cytotoxicity”. Cancer Res. 75 (1): 1781–91. doi:10.1158/0008-5472.CAN-10-1919. PMC 3531552
![Freely accessible Freely accessible]() . PMID 21245102.
. PMID 21245102. - Jump up^ Arnold LH, Kunzelmann S, Webb MR, Taylor IA (Jan 2015). “A continuous enzyme-coupled assay for triphosphohydrolase activity of HIV-1 restriction factor SAMHD1”. Antimicrob Agents Chemother. 59 (1): 186–92. doi:10.1128/AAC.03903-14. PMC 4291348
![Freely accessible Freely accessible]() . PMID 25331707.
. PMID 25331707.
External links
 |
|
| Clinical data | |
|---|---|
| Trade names | Clolar, Evoltra |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607012 |
| License data | |
| Routes of administration |
Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.159.663 |
| Chemical and physical data | |
| Formula | C10H11ClFN5O3 |
| Molar mass | 303.677 g/mol |
| 3D model (JSmol) | |
//////////////////ind 2018, Clofarabine, Nucleotides
C1=NC2=C(N1C3C(C(C(O3)CO)O)F)N=C(N=C2N)Cl


