ELECLAZINE
GS-6615
| Molecular Formula: | C21H16F3N3O3 |
|---|---|
| Molecular Weight: | 415.372 g/mol |
1443211-72-0
4-(pyrimidin-2-ylmethyl)-7-[4-(trifluoromethoxy)phenyl]-2,3-dihydro-1,4-benzoxazepin-5-one
4-(pyrimidin-2-ylmethyl)-7-[4-(trifluoromethoxy)phenyl]-2,3,4,5- tetrahydro-1,4- benzoxazepin-5-one
7-(4-(Trifluoromethoxy)phenyl)-3,4-dihydrobenzo[f][1,4]oxazepin-5(2H)-one
1,4-Benzoxazepin-5(2H)-one, 3,4-dihydro-4-(2-pyrimidinylmethyl)-7-[4-(trifluoromethoxy)phenyl]-
Eleclazine; UNII-PUY08529FK; 1443211-72-0; GS-6615; PUY08529FK; 4-(pyrimidin-2-ylmethyl)-7-(4-(trifluoromethoxy)phenyl)-3,4-dihydrobenzo[f][1,4]oxazepin-5(2H)-on
- Phase III Long QT syndrome
| INGREDIENT | UNII | CAS | |
|---|---|---|---|
| Eleclazine Hydrochloride | 4R1JP3Q4HI | 1448754-43-5 |
Eleclazine has been used in trials studying the treatment of LQT2 Syndrome, Long QT Syndrome, Ischemic Heart Disease, Ventricular Arrhythmia, and Long QT Syndrome Type 3, among others.
In 2015, orphan drug designation was assigned to the product by the FDA for the treatment of congenital long QT syndrome.

- Originator Gilead Sciences
- Class Antiarrhythmics; Ischaemic heart disorder therapies; Pyrimidines; Small molecules; Vasodilators
- Mechanism of Action Sodium channel antagonists
Highest Development Phases
- Phase III Long QT syndrome
- Phase II/III Hypertrophic cardiomyopathy
- Phase II Ventricular arrhythmias
- No development reported Ischaemic heart disorders
Most Recent Events
- 15 Nov 2017 Gilead Sciences presents safety and adverse events data from a phase III trial in Long QT syndrome type 3 at the 90th Annual Scientific Sessions of the American Heart Association (AHA-2017)
- 11 Nov 2017 Efficacy data from the phase II TEMPO trial in Ventricular arrthymmia presented at the 90th Annual Scientific Sessions of the American Heart Association
- 17 Feb 2017 Gilead Sciences terminates a phase II/III trial in Hypertrophic cardiomyopathy in Australia, France, Germany, Israel, Italy, Netherlands, USA and United Kingdom (NCT02291237)
- Gilead Sciences was developing eleclazine (GS-6615), a late sodium current inhibitor, for the potential oral (tablet) treatment of hypertrophic cardiomyopathy and arrhythmias including long QT-3 (LQT3) syndrome.





Long QT syndrome
The late sodium current (INaL) is a component of the fast Na+ current of cardiac myocytes and neurons. Late sodium current in cardiac cells is small compared with the fast component, but it may make a large contribution to sodium loading during each cardiac cycle. Impaired sodium channel function contributes to pathologic increase of the late sodium current, sodium overload, and sodium-induced calcium overload by way of the sodium-calcium exchanger. Calcium overload causes impaired diastolic relaxation, which increases diastolic wall tension, increases myocardial oxygen demand, reduces myocardial blood flow and oxygen supply, microvascular perfusion, and worsens ischemia and angina. Many common neurological and cardiac conditions are associated with abnormal (INaL) augmentation, which contributes to the pathogenesis of both electrical and contractile dysfunction in mammals. Inhibiting the late sodium current can lead to reductions in elevated intracellular calcium levels, which, in turn, may lead to reduced tension in the heart wall and reduced oxygen requirements for the heart muscle. Inhibition of cardiac late sodium current is a strategy used to suppress arrhythmias and sodium -dependent calcium overload associated with myocardial i schemia and heart failures. Thus, compounds that selectively inhibit the iate sodium current (INaL) in mammals may be useful in treating such disease states.
Eleclazine (4-(pyrimidin-2-ylmethyl)-7-(4-(trifluoromethoxy)pheny l)-3,4-dihydrobenzo[b]oxepin-5(2H)-one]; CAS # 144321 1-72-0) is an inhibitor of the late sodium current, Eleclazine is being investigated for the treatment of cardiomyopathy, specifically hypertrophic cardiomyopathy, as well as additional cardiovascular indications, including angina, heart failure, atrial fibrillation (AF), ischemic heart disorders, atrial premature beats (APBs), myocardial isch mia, and arrhythmias.

Eleclazine
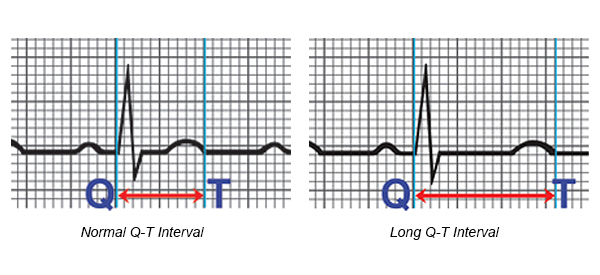
Eleclazine shows a shortening of the QTc interval (the time interval between the start of the Q-wave and the end of T-wave in the electrical cycle of the heart) in patients with QT-3 (LQT3) sydrome. LQTS is a genetic disorder that prolongs the heart’s QTc interval and can cause life-threatening cardiac arrhythmias. Therefore, eleclazine is also being investigated for treatment of long QT syndrome.
Eleclazine may be metabolized in the liver and may be subject to extensive cytochrome P450-mediated oxidative metabolism. Eleclazine is metabolized predominantly by N-dealkylation, and elimination is principally in the bile and gastrointestinal tract. The primary metabolite of eleclazine is GS-623134
Adverse effects associated with eleclazine may include dizziness, dry mouth, nausea, weakness, ringing in ears, tremors, and the like. Additionally, some metabolites of eleclazine, particularly the metabolite GS 623134, may have undesirable side effects.
PATENT
PRODUCT, WO 2013112932, WO 2013006485
WO 2013006463
WO 2013006463 , ( US8962610 ) hold protection in the EU states until 2032 and in US until 2033 with US154 extension.
PATENT
WO 2015017661
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2015017661
Provided herein is a method for reducing the prolongation of the QT interval in a human patient, said method comprising administering to the patient an effective amount of Compound 1:

Example 1: 4-(pyrimidin-2-ylmethyl)-7-(4-(trifluoromethoxy)phenyl)-3,4- dihydrobenzo[f][1,4]oxazepin-5(2H)-one (Compound 1)

To a solution of Compound 1-A (20 g, 0.083 mol, 1 eq.) and Compound 1-B (25 g, 0.15 mol, 1.8 eq.) in DMF (150 mL), NaOH solution (20 mL, 10 M, 5 eq.) was slowly added at room temperature (slightly exothermic) and stirred at r.t. for 10 min, followed by heating at 95 °C for 2 h. After cooling the reaction mixture, ethyl acetate (200 mL) was added and the organic layer was separated. The organics was washed with water (20 mL), brine, dried over sodium sulphate and concentrated.
The residue was dissolved in 1,4-dioxane (50 mL) and to this 4 N HCl in dioxane (50 mL) and cone. HCl ( 2 mL) was added and stirred at room temperature for 4 h, filtered the precipitate, washed with ethyl acetate and dried. Compound 1-C was obtained (30 g) as a light yellow solid.
To the bromide (15 g, 0.04 mol, 1 eq), boronic acid (12.5 g, 0.06 mol, 1.5 eq) and potassium carbonate (22 g, 0.16 mol, 4 eq) in a round bottom flask, solvent (150 mL, toluene/isopropanol/water : 2/1/1) was added and stirred under nitrogen for 10 min. To the above solution the palladium catalyst (1 g, 0.012 mol, 0.02 eq) was added and heated at 85 °C for 2h. The reaction mixture was diluted with ethyl acetate, separated the organic layer and filtered the organic layer through a plug of celite and silica gel and concentrated. Column purification on silica gel using ethyl acetate/hexane as eluent provided Compound 1 (13 g).
To a solution of Compound 1 (26 g) in 1,4-dioxane (25 mL), 4N HCl/dioxane (25 mL) was added followed by cone. HCl (2 mL) and stirred at room temperature for 4h. Solvent was distilled off, dichlorom ethane was added and distilled off and to the residue, ethyl acetate (150 mL) was added and stirred at room temperature overnight and filtered the precipitate, washed with ethyl acetate, hexane and dried under vacuum. Compound 1-HCl obtained (24.8 g) was a white solid.
1H-NMR (CDCl3) 5 8.72 (d, 2H, J= 5.2 Hz), 8.17 (d, 1H, J= 2.4 Hz), 7.59-7.63 (m, 3H), 7.26 (d, 2H, J= 3.2 Hz), 7.22 (t, 1H, J= 4.8 Hz), 7.10 (d, 1H, J= 8.4 Hz), 5.10 (s, 2H), 4.56 (t, 2H, J = 5.0 Hz), 3.77 (t, 2H, J= 5.0 Hz); MS m/z 416.1 (M+H).
PATENT
WO-2018048977
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2018048977&redirectedID=true
Novel deuterated analogs of a substituted oxazepin compounds, particularly eleclazine and their salts, esters, prodrugs and solvates and compositions and combinations comprising them are claimed. Also claim is their use for treating a late sodium current-mediated disorder, such as acute coronary syndrome, angina, congestive heart disease, myocardial infraction, diabetes, ischemic heart disorders, inflammatory diseases and cancers.
EXAMPLE 1- COMPARATIVE
[00297] 4-(pyrimidin-2-ylmethyl)-7-[4-(trifluorome4hoxy)phenyl]-2,3,4,5-tetrahydro-l,4- benzoxazepin-5-one [Eleclazine]

[00299] To a solution of 5-bromo-2-hydroxybenzoate (10 g, 43.28 mmol, 1.00 equiv) in DMA (100 ml.) was added potassium carbonate (9 g, 65, 12 mmol, 1.50 equiv) and 2-chloroacetonitrile (3.4 mL, 1.25 equiv). The resulting suspension was stirred overnight. The solids were filtered out. The filtrate was washed with water. The resulting solution was extracted with ethyl acetate (3 x 50 mL). The organic layers were dried over anhydrous sodium sulfate and concentrated under vacuum to afford 1 1 g (94%) of methyl 5-bromo-2-(cyanomethoxy)benzoate as a white solid, LC-MS: m/z = 270 [M+H]+.
[00300] Step 2: 7-bromo-2,3,4,5-tetrahydro-l,4-benzoxazepin-5-one

[00301] To a solution of 5-bromo-2-(cyanomethoxy)benzoate [Example 1 , Step 1 ] (4 g, 14.81 mmol, 1.00 equiv) in methanol (50 mL) was added saturated aq. NIL (4 mL) and Raney-Ni (2 mL) under a H2 atmosphere. The resulting solution was stirred overnight at room temperature. The catalyst was filtered out. The filtrate was concentrated under vacuum. The residue was purifsed by SiCte chromatography eluted with ethyl acetate/petroleum ether (1 : 1 ) to afford 530 mg (15%) of 7-bromo-2,3,4,5-tetrahydro-l,4-benzoxazepin-5-one as a yellow solid. LC-MS: m/z = 242 [M+H]+.
[00302] Step 3 : 7-bromo-4-(pyrimidin-2-ylmethyl)-2,3,4,5-tetrahydro-l,4-benzoxazepin-5- one

[00303] To a solution of 7-bromo-2,3,4,5-tetrahydro- l ,4-benzoxazepin-5-one [Example 1, Step 2] (530 mg, 2.19 mmol, 1.00 equiv) and 2-(chloromethyl)pyrimidine hydrochloride (650 mg, 3.96 mmol, 1.80 equiv) in DMF (10 mL), was slowly added a NaOH solution (0.55 mL, 10 M, 2.50 equiv), which was stirred at room temperature for 10 min. Then the mixture was stirred at 95°C for 2 h. After cooling the reaction mixture, ethyl acetate (30 mL) was added and the organic layer was separated. The organic layers were washed with water, brine, dried over anhydrous sodium sulfate, and concentrated under vacuum to afford 600 mg (82%) of 7-bromo- 4-(pyrimidin-2-ylmethyl)-2,3,4,5-tetrahydro-l,4-benzoxazepin-5-one as light yellow oil . LC-MS: m/z = 334 [M+H]+.
[00304] Step 4: 4-(pyrimidin-2-ylmethyl)-7-[4-(trifluoromethoxy)phenyl]-2,3,4,5-tetrahydro- 1 ,4-benzoxazepin-5-one

[00305] To a solution of 7-bromo-4-(pyriraidin-2-ylmethyl)-2,3,4,5-tetrahydro-l,4- benzoxaze- pin-5-one [Example 1, Step 3] (277 mg, 0.83 mmol, 1.00 equiv) in Toluene/iPrOH/thO (2: 1 : 1, 4 mL) was added potassium carbonate (459 mg, 3.32 mmol, 4.00 equiv) and [4-(trifluoromethoxy)phenyl]boronic acid (257 mg, 1.25 mmol, 1.50 equiv). The mixture was stirred for 10 min at room temperature. Then Pd(dppf)Ch (12 mg, 0.02 equiv) was added to the solution. The mixture was stirred at 85°C for 2 h. After cooling the reaction mixture, ethyl acetate (30 mL) was added, and the organic layer was separated. The organic layer was washed with water, brine, dried over anhydrous sodium sulfate, and concentrated under vacuum. The crude product was purified by Prep-HPLC with the following conditions: Column, XBridge Prep C18 OBD Column, Sum, 19*150mm; mobile phase, Water (10 mmol/L NH4HCO3) and CH3CN (50,0% CH3CN up to 52.0% in 7 min); Detector, UV 254, 220nra to afford 190 mg (55%) of 4-(pyrimidin-2-ylmethyl)-7-[4-(trifluoromethoxy)phenyl]-2,3,4,5- tetrahydro-1,4- benzoxazepin-5-one as a white solid. LC-MS: m/z = 416 [M+H]+
[00306] 1H NMR (400 MHz, Chloroform-t/) δ 8.75-8.74 (m, 2H), 8.20-8. 19 (m, IH), 7.66- 7,61 (m, 3H), 7,29-7,28 (m, IH), 7.27-7.26 (m, IH), 7.24-7.23 (m, I H), 7.13-7.1 1 (m, IH), 5.12 (s, 2H), 4.60-4.57 (m, 2H), 3.81 -3.78 (m, 2H).
PAPER
Journal of Medicinal Chemistry (2016), 59(19), 9005-9017

Late sodium current (late INa) is enhanced during ischemia by reactive oxygen species (ROS) modifying the Nav 1.5 channel, resulting in incomplete inactivation. Compound 4 (GS-6615, eleclazine) a novel, potent, and selective inhibitor of late INa, is currently in clinical development for treatment of long QT-3 syndrome (LQT-3), hypertrophic cardiomyopathy (HCM), and ventricular tachycardia–ventricular fibrillation (VT–VF). We will describe structure–activity relationship (SAR) leading to the discovery of 4 that is vastly improved from the first generation late INa inhibitor 1(ranolazine). Compound 4 was 42 times more potent than 1 in reducing ischemic burden in vivo (S–T segment elevation, 15 min left anteriorior descending, LAD, occlusion in rabbits) with EC50values of 190 and 8000 nM, respectively. Compound 4 represents a new class of potent late INainhibitors that will be useful in delineating the role of inhibitors of this current in the treatment of patients.
Discovery of Dihydrobenzoxazepinone (GS-6615) Late Sodium Current Inhibitor (Late INai), a Phase II Agent with Demonstrated Preclinical Anti-Ischemic and Antiarrhythmic Properties
Scientific Reports (2017), 7, (1), 1-11.
/////////////////ELECLAZINE, GS-6615, GS 6615, элеклазин , إيليكلازين , 依来克秦 , Phase III, Long QT syndrome, orphan drug designation, Long QT syndrome
C1COC2=C(C=C(C=C2)C3=CC=C(C=C3)OC(F)(F)F)C(=O)N1CC4=NC=CC=N4