
Riamilovir, Triazavirin
Riamilovir sodium dihydrate, CAS 928659-17-0,
Riamilovir CAS: 123606-06-4
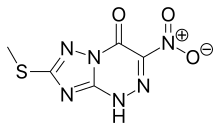
Chemical Formula: C5H4N6O3S
Molecular Weight: 228.19
[1,2,4]Triazolo[5,1-c][1,2,4]triazin-4(1H)-one, 7-(methylthio)-3-nitro- (9CI)
7-(Methylthio)-3-nitro[1,2,4]triazolo[5,1-c][1,2,4]triazin-4(6H)-one
1,2,4]Triazolo[5,1-c][1,2,4]triazin-4(6H)-one, 7-(methylthio)-3-nitro-
7-(methylsulfanyl)-3-nitro[1,2,4]triazolo[5,1-c][1,2,4]triazin- 4(1H)-one
7-thio-substituted-3-nitro-1,2,4-triazolo[5,1-c]-1,2,4-triazin-4(1H)-one
Riamilovir sodium CAS 116061-59-7
Riamilovir sodium dihydrate, CAS 928659-17-0, Triazavirin
Flavivirus infection; Zika virus infection

Zika virus

Flavivirus
Anti-viral drug

http://apps.who.int/medicinedocs/documents/s23256en/s23256en.pdf

Triazavirin (TZV) is a broad-spectrum antiviral drug developed in Russia through a joint effort of Ural Federal University, Russian Academy of Sciences, Ural Center for Biopharma Technologies and Medsintez Pharmaceutical.

It has an azoloazine base structure, which represents a new structural class of non-nucleoside antiviral drugs.[1]
It was originally developed as a potential treatment for pandemic influenza strains such as H5N1, and most of the testing that has been done has focused on its anti-influenza activity.[2][3][4]
However triazavirin has also been found to have antiviral activity against a number of other viruses including tick-borne encephalitis,[5]and is also being investigated for potential application against Lassa fever and Ebola virus disease.[6][7][8][9][10]
Yunona Holdings, was investigating riamilovir sodium dihydrate (triazavirin), a novel nucleoside inhibitor of human influenza virus A and B replication, for the potential oral treatment of influenza virus infection.
In November 2009, the company was seeking to outlicense the drug for development in the EU, presumed to be for use as a prescription medicine .
The Ural Branch of the Russian Academy of Sciences had previously developed, and Yunona Holdings registered and launched, triazavirinin in Russia as an OTC product .
Negative-sense, single-stranded RNA viruses (ssRNA), such as ssRNA viruses belonging to the Order Mononegavirales such as viruses in the Rhabdoviridae family, in particular the Rabies virus, the Filoviridae family, in particular the Ebolavirus, and the Paramyxoviridae family, in particular the Measles virus, other ssRNA viruses belonging to unassigned families such as notably the
Arenaviridae family, the Bunyaviridae family and the Orthomyxoviridae family and other unassigned ssRNA viruses such as notably the Deltavirus, cause many diseases in wildlife, domestic animals and humans. These ssRNA viruses belonging to different families are genetically and antigenically diverse, exhibiting broad tissue tropisms and a wide pathogenic potential.
For example, the Filoviridae viruses belonging to the Order
Mononegavirales, in particular the Ebolaviruses and Marburgviruses, are among the most lethal and most destructive viruses in the world. Filoviridae viruses are of particular concern as possible biological weapons since they have the potential for aerosol dissemination and weaponization.
The Ebolavirus includes five species: the Zaire, Sudan, Reston, Ta‘i Forest and Bundibugyo Ebolaviruses. In particular the Zaire, Sudan and Bundibugyo Ebolavirus cause severe, often fatal, viral hemorraghic fevers in humans and nonhuman primates.
For more than 30 years, the Ebolavirus has been associated with periodic episodes of hemorrhagic fever in Central Africa that produce severe disease in
infected patients. Mortality rates in outbreaks have ranged from 50% for the Sudan species of the Ebolavirus to up to 90% for the Zaire species of the Ebolavirus ((Sanchez et al., Filoviridae: Marburg and Ebola Viruses, in Fields Virology, pages 1409-1448 (Lippincott Williams & Wilkins, Philadelphia)). In November 2007, during an outbreak in the Bundibugyo district of Uganda, near the border with the Democratic Republic of the Congo the fifth species of the Ebolavirus was discovered, the Bundibugyo species. Said outbreak resulted in a fatality rate of about 25% (Towner et al., PLoS Pathog., 4(11 ) :e1000212 (2008)). The Zaire species of the Ebolavirus has also decimated populations of wild apes in this same region of Africa (Walsh et al., Nature, 422:611-614 (2003)).
When infected with the Ebolavirus, the onset of illness is abrupt and is characterized by high fever, headaches, joint and muscle aches, sore throat, fatigue, diarrhea, vomiting, and stomach pain. A rash, red eyes, hiccups and internal and external bleeding may be seen in some patients. Within one week of becoming infected with the virus, most patients experience chest pains and multiple organ failure, go into shock, and die. Some patients also experience blindness and extensive bleeding before dying.
Another example of a negative sense single-stranded RNA envelope virus is the Morbilllivirus such as the Measles virus which is associated with Measles and the Lyssavirus such as the Rabies virus.
The Lyssavirus, belonging to the family Rhabdoviridae, includes eleven recognized species, in particular the Rabies virus which is known to cause Rabies. Rabies is an ancient disease with the earliest reports possibly dated to the Old World before 2300 B.C and remains a world health threat due to remaining lack of effective control measures in animal reservoir populations and a widespread lack of human access to vaccination. The Rabies virus is distributed worldwide among mammalian reservoirs including carnivores and bats. Each year there are many reported cases of transmission of the Rabies virus from animals to humans (e.g. by an animal bite). More than 50,000 people annually die of Rabies, particularly in Asia and Africa.
Thus, there remains a need for antiviral compounds which are effective for use in the treatment of the ssRNA virus infections different from the Influenza A and Influenza B virus infections
SYNTHESIS CONTRUCTED WITH 3 ARTICLES AS BELOW
RU 2340614 C2 20081210,
e-EROS Encyclopedia of Reagents for Organic Synthesis, 1-7; 2009,
European Journal of Medicinal Chemistry, 113, 11-27; 2016


Khimiya Geterotsiklicheskikh Soedinenii (1989), (2), 253-7.
Khimiya Geterotsiklicheskikh Soedinenii (1992), (11), 1555-9.
Zhurnal Organicheskoi Khimii (1996), 32(5), 770-776
PAPER
Russian Journal of Organic Chemistry (Translation of Zhurnal Organicheskoi Khimii) (2002), 38(2), 272-280.
https://link.springer.com/article/10.1023%2FA%3A1015538322029
Adamantylation of 3-Nitro- and 3-Ethoxycarbonyl-1,2,4-triazolo[5,1-c]-1,2,4-triazin-4-ones
Abstract
Reaction of 3-nitro- and 3-ethoxycarbonyl-1,2,4-triazolo[5,1-c]-1,2,4-triazin-4-ones with 1-adamantanol (or 1-adamantyl nitrate) in concentrated sulfuric acid or with 1-bromoadamantane in sulfolane affords N-adamantyl derivatives. The adamantylation of 3-nitro-1,4-dihydro-7H-1,2,4-triazolo[5,1-c]-1,2,4-triazin-4-one yields a mixture of N8– and N1-isomers that undergo interconversion in concentrated sulfuric acid along intermolecular mechanism.
PATENT
RU 2340614 C2 20081210,
PAPER
Russian Chemical Bulletin (2010), 59(1), 136-143.
Synthesis and antiviral activity of nucleoside analogs based on 1,2,4-triazolo[3,2-c][1,2,4]triazin-7-ones
Abstract
Nucleoside analogs containing hydroxybutyl, hydroxyethoxymethyl, allyloxymethyl, and propargyloxymethyl fragments were synthesized based on 1,2,4-triazolo[3,2-c][1,2,4]triazin-7-ones isosteric to purine bases. Some of the compounds obtained inhibit in vitro reproduction of influenza and respiratory syncytial virus infection.
PATENT
WO 2015117016
PAPER
Chemistry of Heterocyclic Compounds (New York, NY, United States) (2015), 51(3), 275-280.
https://link.springer.com/article/10.1007%2Fs10593-015-1695-4
The nucleophilic susbstitution of nitro group in [1,2,4]triazolo[5,1-c][1,2,4]triazinones upon treatment with cysteine and glutathione was studied as a model for the interaction with thiol groups of virus proteins, which mimics the metabolic transformations of antiviral drug Triazavirin® and its derivatives.
Nucleophilic substitution of nitro group in nitrotriazolotriazines as a model of potential interaction with cysteine-containing proteins
PATENT
Example 1 : One pot synthesis of the sodium salt of 7-methylthio-3-nitro [1 ,
2, 4] triazolo [5,1 -c] [1, 2, 4] triazin -4 (1H)-one
Step 1 : Diazotization of compound (B): A solution (solution [1], herein after) was prepared of 5.8 g (0.05 ) of 5-amino-3-mercapto-1 ,2,4-triazole in 6.7 ml of nitric acid (15 M) and 12 ml of water. Said solution [1] was refrigerated to -7°C . Then a 40% sodium nitrite solution was added to the solution [1] in portions of 0.5 mL to obtain a total amount of sodium nitrite equal to 3.8 g in the mixture.
Step 2: Condensation of diazonium compound with an a-nitroester:
To the resulting diazonium salt of step 1 , 8.54 ml of diethyl nitromaionate was added. After holding for five minutes, a cooled solution of sodium hydroxide was slowly added dropwise to the reaction mixture until the pH was between pH 9 and pH 10 (solution [2], herein after). The resulting solution [2] was stirred at 0°C for 1 hour and at room temperature for 2 hours.
Step 3: alkylation: To the solution [2] of step 2, 6.23 ml (0.1 moi) of methyl iodide was added. The mixture was stirred for 1 hour at room temperature and filtered. The resulting precipitate was successively crystallized from water and dried in air. The reaction scheme is depicted below in Scheme 1.

SCHEME 1
The yield was 9.87 g (69%).
Physical and chemical characteristics of the sodium salt of 7-methylthio-3-nitro
[1, 2, 4] triazolo [5,1-c] [1, 2, 4] triazin -4 (1H)-one sodium salt: yellow crystalline powder, soluble in water, acetone, dimethylsulfoxide, dimethylformamide. insoluble in chloroform; Tmelt = 300°C, H NMR spectrum, δ, ppm, solvent DMSO-d6: 2.62 (3H, s, SCH3); IR spectrum, n, cm“1: 3535 (OH), 1649 (C=0), 1505 (N02), 1367 (N02); found.. %: C – 20.86, H 2.51 , N 29.28;
C5H;N6Na05S; Calculated, %: C – 20.98, H 2.47, N 29.36.
Example 2: Synthesis of the sodium salt of 7-methylthio-3-nitro [1, 2, 4] triazolo [5,1-c] [1, 2, 4] triazin -4 (1H)-one sodium salt
In this example the synthesis comprises 3 steps: in the first step 5-amino-3-mercapto-1.2,4-triazole (i.e. compound (B)) was prepared by condensation of aminoguanidine with a thio-derivative (thio ester) of formic acid, HC(=0)S-R, wherein -R was: methyl. In the second step 5-amino-3-mercapto-1 , 2,4-triazole was converted to the corresponding diazonium salt. In the third step this diazonium salt was reacted with an a-nitroester, 2-nitroacetoacetic ester, to form the 7-methylthio-3-nitro [1, 2, 4] triazolo [5,1-c] [1, 2, 4] triazin -4 (1H)-one. The different steps are explained in more detail below.
Step 1 : Synthesis of compound (B): In a reaction flask equipped with a stirrer, reflux condenser, under inert gas (nitrogen, argon), 20 g (0.1 mol) of aminoguanidine and 7.6 g (0.1 mol) methylthio-formate was added to 400 ml of absolute pyridine. The reaction mixture was boiled for 4 hours at 115°C.
Subsequently the reaction mixture was transferred into distilled water and washed several times with water. The washed mixture was dried over a Nutsche filter under vacuum. Recrystallization was carried out from ethanol. The reaction scheme is depicted below in Scheme 2.

SCHEME 2
The yield was 19.3 g (70%)
Step 2: Diazotation of compound (B): A solution (solution [3], herein after) was prepared of 26 g (0.1 mol) of 5-amino-3-mercapto-1 ,2,4-triazole (as obtained in step 1) in 32 ml of nitric acid (0.1 mol) and 200 ml of water. The solution was mixed and cooled to -5°C. In a separate recipient, a 0.1 M solution of sodium nitrite was prepared by dissolving 16 g of sodium nitrite in 100 ml of water. The sodium nitrite solution was put in the freezer until there was ice formation and subsequently the ice was crushed. Thereafter, the solution [3] and the sodium nitrite crushed ice were transferred into a 1 L reactor and stirred for 1 hour while the reactor temperature was kept at 0°C. The low temperature and the fact that the two reaction components are in different phases (i.e. liquid and solid) ensured a slow gradual progress of diazotization reaction at the phase interface. The end of the diazotization process was controlled by a iodine starch test (proof of the absence of sodium nitrite in a free state).
The rea

SCHEME 3
Step 3: Condensation of the diazonium compound with an α-nitroester: A solution (solution [4], herein after) was prepared by mixing 17.5 g of methyl 2- nitro-acetoacetate in 300 mL of isopropanoi. The solution [4] was mixed with the diazonium salt of step 2. The mixture was cooled to 0°C. At 0°C, a 10% sodium hydroxide solution was added to the reaction mixture (to neutralize residual nitrite and acetate) until there was a marked alkaline reaction (pH between 8 and 9). The temperature was controlled and was kept below +5°C. The resulting mixture was stirred for 1 hour. The precipitate was filtered off and dried in air. The yield was 78%.
The reaction scheme is depicted in Scheme 4

SCHEME 4
Example 3: Synthesis of the sodium salt of 7-methylthio-3-nitro [1, 2, 4] triazolo [5,1-c] [1, 2, 4] triazin -4 (1H)-one
The synthesis of the sodium salt of 7-methylthio-3-nitro-1 ,2,4-triazolo [5,1-c]-1 ,2,4-triazin-7-one may be carried out as in Example 2, only in step 2 the aqueous alcohol solution is replaced by an alcohol with alkali (such as sodium hydroxide). The yield of the antiviral compound (A) (sodium salt of 7-methylthio-3-nitro [1 2, 4] triazolo [5,1-c] [1 , 2. 4] triazin -4 (1 H)-one) may increase to 83%. The reaction scheme is depicted below in Scheme 5:

SCHEME 5
PATENT
Process for the preparation of 7-thio-substituted-3-nitro-1,2,4-triazolo[5,1-c]-1,2,4-triazin-4(1H)-one i.e. riamilovir sodium dihydrate is claimed. Also claimed are use of triazolo compounds for the treatment of ssRNA virus infections such as Zika virus and flavivirus, ssRNA viruses different from the Influenza A and Influenza B viruses and compositions comprising them. Along with concurrently published WO2017144709 claiming similar derivatives. Represents new area of interest from Doring International Gmbh and the inventors on this moiety.
Example 1 : One pot synthesis of the sodium salt of 7-methylthio-3-nitro [1, 2, 4] triazolo [5,1 -cj [1, 2, 4] triazin -4 (1H)-one
Step 1: Diazotization of compound (B): A solution (solution [1], herein after) was prepared of 5.8 g (0.05 M) of 5-amino-3-mercapto-1 ,2,4-triazole in 6.7 ml of nitric acid (15 M) and 12 ml of water. Said solution [1] was refrigerated to -7°C . Then a 40% sodium nitrite solution was added to the solution [1] in portions of 0.5 mL to obtain a total amount of sodium nitrite equal to 3.8 g in the mixture.
Step 2: Condensation of diazonium compound with an a-nitroester: To the resulting diazonium salt of step 1 , 8.54 ml of diethyl nitromalonate was added. After holding for five minutes, a cooled solution of sodium hydroxide was slowly added dropwise to the reaction mixture until the pH was between pH 9 and pH 10 (solution [2], herein after). The resulting solution [2] was stirred at 0°C for 1 hour and at room temperature for 2 hours.
Step 3: alkylation: To the solution [2] of step 2, 6.23 ml (0.1 mol) of methyl iodide was added. The mixture was stirred for 1 hour at room temperature and
filtered. The resulting precipitate was successively crystallized from water and dried in air. The reaction scheme is depicted below in Scheme 1.

SCHEME 1
The yield was 9.87 g (69%).
Physical and chemical characteristics of the sodium salt of 7-methylthio-3-nitro
[1, 2, 4] triazolo [5,1-c] [1, 2, 4] triazin -4 (1H)-one sodium salt: yellow crystalline powder, soluble in water, acetone, dimethylsulfoxide, dimethylformamide, insoluble in chloroform; Tmei, = 300°C, 1H NMR spectrum, δ, ppm, solvent DMSO-d6: 2.62 (3H, s, SCH3); IR spectrum, n, cm“1: 3535 (OH), 1649 (CO), 1505 (N02), 1367 (N02); found, %: C – 20.86, H 2.51 , N 29.28; C5H7N6Na05S; Calculated, %: C – 20.98, H 2.47, N 29.36.
Example 2: Synthesis of the sodium salt of 7-methylthio-3-nitro [1, 2,
4] triazolo [5,1-c] [1, 2, 4] triazin -4 (1H)-one sodium salt
In this example the synthesis comprises 3 steps: in the first step 5-amino-3-mercapto-1 ,2,4-triazole (i.e. compound (B)) was prepared by condensation of aminoguanidine with a thio-derivative (thio ester) of formic acid, HC(=0)S-R, wherein -R was: methyl. In the second step 5-amino-3-mercapto-1 ,2,4-triazole was converted to the corresponding diazonium salt. In the third step this diazonium salt was reacted with an a-nitroester, 2-nitroacetoacetic ester, to form the 7-methylthio-3-nitro [1, 2, 4] triazolo [5,1-c] [1, 2, 4] triazin -4 (1H)-one. The different steps are explained in more detail below.
Step 1 : Synthesis of compound (B): In a reaction flask equipped with a stirrer, reflux condenser, under inert gas (nitrogen, argon), 20 g (0.1 mo!) of
aminoguanidine and 7.6 g (0.1 mol) methylthio-formate was added to 400 ml of absolute pyridine. The reaction mixture was boiled for 4 hours at 115°C. Subsequently the reaction mixture was transferred into distilled water and washed several times with water. The washed mixture was dried over a Nutsche filter under vacuum. Recrystallization was carried out from ethanol. The reaction scheme is depicted below in Scheme 2.

SCHEME 2
The yield was 19.3 g (70%)
Step 2: Diazotation of compound (B): A solution (solution [3], herein after) was prepared of 26 g (0.1 mol) of 5-amino-3-mercapto-1 ,2,4-triazole (as obtained in step 1 ) in 32 ml of nitric acid (0.1 mol) and 200 ml of water. The solution was mixed and cooled to -5°C. In a separate recipient, a 0.1 M solution of sodium nitrite was prepared by dissolving 16 g of sodium nitrite in 100 ml of water. The sodium nitrite solution was put in the freezer until there was ice formation and subsequently the ice was crushed. Thereafter, the solution [3] and the sodium nitrite crushed ice were transferred into a 1 L reactor and stirred for 1 hour while the reactor temperature was kept at 0°C. The low temperature and the fact that the two reaction components are in different phases (i.e. liquid and solid) ensured a slow gradual progress of diazotization reaction at the phase interface. The end of the diazotization process was controlled by a iodine starch test (proof of the absence of sodium nitrite in a free state).
The rea

SCHEME 3
Step 3: Condensation of the diazonium compound with an a-nitroester: A solution (solution [4], herein after) was prepared by mixing 17.5 g of methyl 2-nitro-acetoacetate in 300 mL of isopropanol. The solution [4] was mixed with the diazonium salt of step 2. The mixture was cooled to 0°C. At 0°C, a 10% sodium hydroxide solution was added to the reaction mixture (to neutralize residual nitrite and acetate) until there was a marked alkaline reaction (pH between 8 and 9). The temperature was controlled and was kept below +5°C. The resulting mixture was stirred for 1 hour. The precipitate was filtered off and dried in air. The yield was 78%.
The reaction scheme is depicted in Scheme 4

SCHEME 4
Example 3: Synthesis of the sodium salt of 7-methylthio-3-nitro [1, 2, 4] triazolo [5,1-c] [1, 2, 4] triazin -4 (1H)-one
The synthesis of the sodium salt of 7-methy I th io-3-nitro- 1 ,2, 4-triazolo [5,1-c]-1 ,2,4-triazin-7-one may be carried out as in Example 2, only in step 2 the aqueous alcohol solution is replaced by an alcohol with alkali (such as sodium hydroxide). The yield of the antiviral compound (A) (sodium salt of 7-methylthio-3-nitro [1 2, 4] triazolo [5,1-c] [1 , 2, 4] triazin -4 (1 H)-one) may increase to 83%.
The reaction scheme is depicted below in Scheme 5:

SCHEME 5
References
- Jump up^ Rusinov VL, Sapozhnikova IM, Ulomskii EN, Medvedeva NR, Egorov VV, Kiselev OI, Deeva EG, Vasin AV, Chupakhin ON. Nucleophilic substitution of nitro group in nitrotriazolotriazines as a model of potential interaction with cysteine-containing proteins. Chemistry of Heterocyclic Compounds 2015;51(3):275-280. doi 10.1007/s10593-015-1695-4
- Jump up^ Loginova SIa, Borisevich SV, Maksimov VA, Bondarev VP, Kotovskaia SK, Rusinov VL, Charushin VN. Investigation of triazavirin antiviral activity against influenza A virus (H5N1) in cell culture. (Russian) Antibiotiki i Khimioterapiia. 2007;52(11-12):18-20. PMID 19275052
- Jump up^ Karpenko I, Deev S, Kiselev O, Charushin V, Rusinov V, Ulomsky E, Deeva E, Yanvarev D, Ivanov A, Smirnova O, Kochetkov S, Chupakhin O, Kukhanova M. Antiviral properties, metabolism, and pharmacokinetics of a novel azolo-1,2,4-triazine-derived inhibitor of influenza A and B virus replication. Antimicrobial Agents and Chemotherapy. 2010 May;54(5):2017-22. doi: 10.1128/AAC.01186-09 PMID 20194696
- Jump up^ Kiselev OI, Deeva EG, Mel’nikova TI, Kozeletskaia KN, Kiselev AS, Rusinov VL, Charushin VN, Chupakhin ON. A new antiviral drug Triazavirin: results of phase II clinical trial. (Russian). Voprosy Virusologii. 2012 Nov-Dec;57(6):9-12. PMID 23477247
- Jump up^ Loginova SIa, Borisevich SV, Rusinov VL, Ulomskiĭ UN, Charushin VN, Chupakhin ON. Investigation of Triazavirin antiviral activity against tick-borne encephalitis pathogen in cell culture. (Russian). Antibiotiki i Khimioterapiia. 2014;59(1-2):3-5. PMID 25051708
- Jump up^ “Target: Ebola”. Pravda. Retrieved 18 January 2015.
- Jump up^ “Yekaterinburg pharmacies to sell domestic antiviral drug”. Retrieved 18 January 2015.
- Jump up^ “Ebola crisis: Vaccine ‘too late’ for outbreak. BBC News, 17 October 2014”. BBC News.
- Jump up^ Kukil Bora. Russia Will Begin Testing Triazavirin, Used For Lassa Fever, And Other Drugs On Ebola: Health Ministry. International Business Times, 12 November 2014
- Jump up^ Darya Kezina. New antiviral drug from Urals will help fight Ebola and other viruses. Russia Beyond the Headlines, 12 November 2014
 |
|
| Legal status | |
|---|---|
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ECHA InfoCard | 100.217.074 |
| Chemical and physical data | |
| Formula | C5H4N6O3S |
| Molar mass | 228.189 |
| 3D model (JSmol) | |
///////////riamilovir sodium dihydrate, Riamilovir , ANTIVIRAL, Triazavirin, Flavivirus infection, Zika virus infection
O=C1N2C(NN=C1[N+]([O-])=O)=NC(SC)=N2
Filed under: Phase2 drugs Tagged: antiviral, Flavivirus infection, Riamilovir, riamilovir sodium dihydrate, Triazavirin, Zika virus infection
![[1,2,4]Triazolo[5,1-c][1,2,4]triazin-4(1H)-one, 7-(methylthio)-3-nitro-.png](http://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?cid=3113817&t=l)

