


(1R,2S)-2-(Aminomethyl)-N,N-diethyl-1 phenylcyclobutanecarboxamide (19)
1 H NMR (CDCl3) δ 7.36–7.33 (m, 4H), 7.25–7.21 (m, 1H), 3.51–3.43 (qd, J = 13.8 Hz, 6.8 Hz, 1H), 3.15–2.87 (m, 7H), 2.81–2.72 (m, 2H), 2.23–2.14 (m, 1H), 2.04–1.97 (m, 1H), 1.62 (tdd, J = 10.5 Hz, 5.7 Hz, 2.6 Hz, 1H), 1.07 (t, J = 7.1 Hz, 3H), 0.35 (t, J = 7.1 Hz, 3H) ppm;
13C NMR (CDCl3) δ 172.7, 143.3, 128.8, 126.4, 125.3, 54.6, 44.4, 42.4, 41.0, 39.5, 31.1, 19.0, 12.2, 12.0 ppm;
IR (neat) 3364, 1622, 1437, 905, 728 cm−1 ;
[α] 20 D +1.5 (c 0.5, CHCl3) (lit.5 [α]D +0.84);
ESI-MS (ES+ ) 261 [M + H]+ ; HRMS m/z calcd for C16H25N2O: 261.1958, found: 261.1961;
chiral HPLC (CHIRALCEL OJ-RH 150 × 4.6 mm, H2O/MeOH 35 : 65, flow rate 1 mL min−1 , detection at 254 nm), tmajor = 8.5 min, tminor = 6.7 min, er 95 : 5. Of note, compound 19 was acetylated with acetic anhydride/NEt3 prior to HPLC analysis.
5 S. Cuisiat, A. Newman-Tancredi, O. Vitton and B. Vacher, WO patent, 112597, 2010
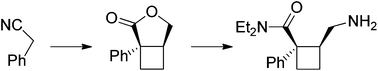
Enantioselective synthesis of a cyclobutane analogue of Milnacipran
DOI: 10.1039/C7QO00140A, Research Article
An optically active cyclobutane analogue of Milnacipran was synthesized from phenylacetonitrile, and its cis-stereochemistry was controlled by an epimerization step.
Enantioselective synthesis of a cyclobutane analogue of Milnacipran
aService de Chimie Bioorganique et de Marquage (SCBM), CEA, Université Paris-Saclay, 91191 Gif-sur-Yvette, France
Abstract
The asymmetric synthesis of a cyclobutane analogue of the antidepressant drug Milnacipran is reported. The optically active derivative incorporates a central cyclobutane ring in lieu of the cyclopropane unit classically found in Milnacipran. The two stereogenic centres borne by the cyclobutane were sequentially installed starting from phenylacetonitrile.
Filed under: PROCESS, Uncategorized Tagged: milnacipran