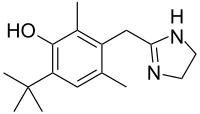
Oxymetazoline
1491-59-4 CAS NUMBER
- Molecular FormulaC16H24N2O
- Average mass260.375 Da
Oxymetazoline is a selective α1 adrenergic receptor agonist and α2 adrenergic receptorpartial agonist. It is a topical decongestant, used in the form of oxymetazoline hydrochloride. It was developed from xylometazoline at E. Merck Darmstadt by Fruhstorfer in 1961.[1]Oxymetazoline is generally available as a nasal spray.

Oxymetazoline HCl; CAS 2315-02-8
Oxymetazoline hydrochloride is a vasoconstrictor. Chemically it is 3-[(4,5-dihydro-1H-imidazol-2-yl)methyl]6-(1,1,-dimethylethyl)-2,4-dimethylphenolmono-hydrochloride. Its molecular weight is 296.8 for the hydrochloride salt and 260.4 for the free base. It is freely soluble in water and ethanol and has a partition coefficient of 0.1 in octanol/water. Its structural formula is:
 |
Medical uses
Oxymetazoline is available over-the-counter as a topical decongestant in the form of oxymetazoline hydrochloride in nasal sprays such as Afrin, Operil, Dristan, Dimetapp, oxyspray, Facimin, Nasivin, Nostrilla, Sudafed OM, Vicks Sinex, Zicam, SinuFrin, and Mucinex Full Force.[2]
Due to its vasoconstricting properties, oxymetazoline is also used to treat nose bleeds[3][4]and eye redness due to minor irritation (marketed as Visine L.R. in the form of eye drops).[5]
Company:
Allergan
Approval Status:
Approved January 2017
Specific Treatments:
facial erythema associated with rosacea
Therapeutic Areas
Dermatology
Find Related Trials for The Following Conditions
Rosacea
General Information
Rhofade (oxymetazoline hydrochloride) is an alpha1A adrenoceptor agonist. Oxymetazoline acts as a vasoconstrictor.
Rhofade is spccifically indicated for the topical treatment of persistent facial erythema associated with rosacea in adults.
Rhofade is supplied as a cream for topical administration. Apply a pea-sized amount of Rhofade cream, once daily in a thin layer to cover the entire face (forehead, nose, each cheek, and chin) avoiding the eyes and lips. Wash hands immediately after applying Rhofade cream.

Side effects and special considerations
Rebound congestion
It is recommended that oxymetazoline not be used for more than three days, as rebound congestion, or rhinitis medicamentosa, may occur.[6] Patients who continue to use oxymetazoline beyond this point may become dependent on the medication to relieve their chronic congestion.
Effects of benzalkonium chloride
Some studies have found that benzalkonium chloride, a common additive to oxymetazoline nasal sprays, may damage nasal epithelia and exacerbate rhinitis medicamentosa. However, the majority of studies find benzalkonium chloride to be a safe preservative.[7]
Use in pregnancy
The Food and Drug Administration places oxymetazoline in category C, indicating risk to the fetus cannot be ruled out. While it has been shown that a single dose does not significantly alter either maternal or fetal circulation,[8] this subject has not been studied extensively enough to draw reliable conclusions.
Overdose
If accidentally ingested, standard methods to remove unabsorbed drugs should be considered.[clarification needed] There is no specific antidote for oxymetazoline, although its pharmacological effects may be reversed by α adrenergic antagonists such as phentolamine. In the event of a possibly life-threatening overdose (such as a hypertensive crisis), benzodiazepines should be considered to decrease the likelihood of seizures and convulsions, as well as reduce anxiety and to lower blood pressure. In children, oxymetazoline may produce profound central nervous system depression due to stimulation of central α2receptors and imidazoline receptors, much like clonidine.
Pharmacology
Mechanism of action
Oxymetazoline is a sympathomimetic that selectively agonizes α1 and, partially, α2 adrenergic receptors.[9] Since vascular beds widely express α1 receptors, the action of oxymetazoline results in vasoconstriction. In addition, the local application of the drug also results in vasoconstriction due to its action on endothelial postsynaptic α2 receptors; systemic application of α2 agonists, in contrast, causes vasodilation because of centrally-mediated inhibition of sympathetic tone via presynaptic α2 receptors.[10] Vasoconstriction of vessels results in relief of nasal congestion in two ways: first, it increases the diameter of the airway lumen; second, it reduces fluid exudation from postcapillary venules.[11] It can reduce nasal airway resistance (NAR) up to 35.7% and nasal mucosal blood flow up to 50%.[12]
Pharmacokinetics
Imidazolines are sympathomimetic agents, with primary effects on α adrenergic receptors and little if any effect on β adrenergic receptors. Oxymetazoline is readily absorbed orally. Effects on α receptors from systemically absorbed oxymetazoline hydrochloride may persist for up to 7 hours after a single dose. The elimination half-life in humans is 5–8 hours. It is excreted unchanged both by the kidneys (30%) and in feces (10%).
History
The oxymetazoline brand Afrin was first sold as a prescription medication in 1966. After finding substantial early success as a prescription medication, it became available as an over-the-counter drug in 1975. Schering-Plough did not engage in heavy advertising until 1986.[13]From the late 1980s to mid 1990s, Afrin featured in many notable television advertisements. Some of these commercials showed men, women, and children using other brands of nasal sprays, and then standing upside down or hanging upside down from playground equipment to prevent their nasal spray from dripping out. This was juxtaposed with Afrin users having no problems.
Society and culture
Brand names
Brand names include Afrin, Dristan, Nasivin, Nezeril, Nostrilla, Logicin, Vicks Sinex, Visine L.R., Sudafed OM, Zicam, SinuFrin and Mucinex Sinus-Max.


References
- Jump up^ German Patent 1,117,588
- Jump up^ “Oxymetazoline: Drug Information Provided by Lexi-Comp: Merck Manual Professional”. Merck.com. Retrieved 2013-04-15.
- Jump up^ Katz, Robert I.; Hovagim, Alec R.; Finkelstein, Harvey S.; Grinberg, Yair; Boccio, Remigio V.; Poppers, Paul J. (1990). “A comparison of cocaine, lidocaine with epinephrine, and oxymetazoline for prevention of epistaxis on nasotracheal intubation”. Journal of Clinical Anesthesia. 2 (1): 16–20. doi:10.1016/0952-8180(90)90043-3. PMID 2310576.
- Jump up^ Krempl, G. A.; Noorily, A. D. (1995). “Use of oxymetazoline in the management of epistaxis”. The Annals of otology, rhinology, and laryngology. 104 (9 Pt 1): 704–6.PMID 7661519.
- Jump up^ “VISINE® Original Red Eye Drops | VISINE® products”. Visine.com. Retrieved2013-04-15.
- Jump up^ Ramey, J. T.; Bailen, E; Lockey, R. F. (2006). “Rhinitis medicamentosa”. Journal of investigational allergology & clinical immunology. 16 (3): 148–55. PMID 16784007.
- Jump up^ Marple, B; Roland, P; Benninger, M (2004). “Safety review of benzalkonium chloride used as a preservative in intranasal solutions: An overview of conflicting data and opinions”. Otolaryngology – Head and Neck Surgery. 130 (1): 131–41.doi:10.1016/j.otohns.2003.07.005. PMID 14726922.
- Jump up^ Rayburn, W. F.; Anderson, J. C.; Smith, C. V.; Appel, L. L.; Davis, S. A. (1990).“Uterine and fetal Doppler flow changes from a single dose of a long-acting intranasal decongestant”. Obstetrics and gynecology. 76 (2): 180–2. PMID 2196495.
- Jump up^ Westfall Thomas C, Westfall David P, “Chapter 6. Neurotransmission: The Autonomic and Somatic Motor Nervous Systems” (Chapter). Brunton LL, Lazo JS, Parker KL: Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 11e: http://www.accessmedicine.com/content.aspx?aID=954433.
- Jump up^ Biaggioni Italo, Robertson David, “Chapter 9. Adrenoceptor Agonists & Sympathomimetic Drugs” (Chapter). Katzung BG: Basic & Clinical Pharmacology, 11e: http://www.accessmedicine.com/content.aspx?aID=4520412.
- Jump up^ Widdicombe, John (1997). “Microvascular anatomy of the nose”. Allergy. 52 (40 Suppl): 7–11. doi:10.1111/j.1398-9995.1997.tb04877.x. PMID 9353554.
- Jump up^ Bende, M.; Löth, S. (2007). “Vascular effects of topical oxymetazoline on human nasal mucosa”. The Journal of Laryngology & Otology. 100 (3): 285–8.doi:10.1017/S0022215100099151. PMID 3950497.
- Jump up^ Dougherty, Phillip H. (20 October 1986). “Advertising; Afrin Goes After Users Of Nasal Decongestants”. The New York Times. The New York Times Company. Retrieved 2015-03-30.
 |
|
| Clinical data | |
|---|---|
| Trade names | Afrin, Ocuclear, Drixine |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Dependence liability |
Moderate |
| Routes of administration |
Intranasal |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Kidney (30%), fecal (10%) |
| Biological half-life | 5–6 hours |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.014.618 |
| Chemical and physical data | |
| Formula | C16H24N2O |
| Molar mass | 260.375 g·mol−1 |
| 3D model (Jmol) | |
| Melting point | 301.5 °C (574.7 °F) |
/////////Oxymetazoline, оксиметазолин , أوكسيميتازولين , 羟甲唑啉 , Rhofade, oxymetazoline hydrochloride, alpha1A adrenoceptor agonist, vasoconstrictor
CC1=CC(=C(C(=C1CC2=NCCN2)C)O)C(C)(C)C.Cl
Filed under: FDA 2017, Uncategorized Tagged: alpha1A adrenoceptor agonist, 羟甲唑啉, оксиметазолин, Oxymetazoline, oxymetazoline hydrochloride, Rhofade, vasoconstrictor, أوكسيميتازولين

