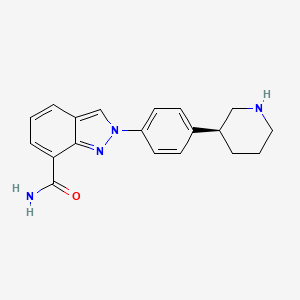
MK-4827,(S)-2-(4-(piperidin-3-yl)phenyl)-2H-indazole-7-carboxaMide
1038915-60-4 CASfree form
1038915-64-8 CAS HYDROCHLORIDE
1613220-15-7 cas TOSYLATE MONOHYDRATE

| 1038915-73-9 TOSYLATE |
MK-4827(Niraparib) tosylate is a selective inhibitor of PARP1/PARP2 with IC50 of 3.8 nM/2.1 nM; with great activity in cancer cells with mutant BRCA-1 and BRCA-2; >330-fold selective against PARP3, V-PARP and Tank1.
IC50 value: 3.8 nM/2.1 nM( PARP1/2) [1]
Target: PARP1/2
in vitro: MK-4827 displays excellent PARP 1 and 2 inhibition with IC(50) = 3.8 and 2.1 nM, respectively, and in a whole cell assay, it inhibits PARP activity with EC(50) = 4 nM and inhibits proliferation of cancer cells with mutant BRCA-1 and BRCA-2 with CC(50) in the 10-100 nM range [1].
in vivo: MK-4827 is well tolerated in vivo and demonstrates efficacy as a single agent in a xenograft model of BRCA-1 deficient cancer [1]. In addition, MK-4827 strongly enhances the effect of radiation on a variety of human tumor xenografts, both p53 wild type and p53 mutant. The enhancement of radiation response is observed in clinically relevant radiation-dose fractionation schedules. The therapeutic window during which time MK-4827 interacts with radiation lasts for several hours. These biological attributes make translation of this therapeutic combination treatment feasible for translation to the treatment of a variety of human cancers [2].
MERCK

TESARO

Niraparib (originally MK-4827)[1] is an orally active[2] small molecule PARP inhibitor being developed (by Tesaro) to treat ovarian cancer.
It is an inhibitor of PARP1 and PARP2.[3]
Niraparib is due to be submitted for FDA approval (for maintenance therapy in ovarian cancer) later in 2016.[4]
Chemically, MK-4827 is C19H20N4O[5] (ignoring a possible tosylate group).[6]
A 2012 study found that PARP inhibitors exhibit cytotoxic effects not based solely on their enzymatic inhibition of PARP, but by their trapping of PARP on damaged DNA, and the strength of this trapping activity was ordered niraparib >> olaparib >> veliparib.[7]

![str1]() NMR CD3OD
NMR CD3OD
Clinical trials
It has undergone a phase III trial for ovarian cancer.[8] It is reported that the primary endpoint (progression-free survival, PFS) was met.[4] Patients with and without a BRCA mutation both showed longer PFS.[4]
As of June 2016 seven clinical trials have been registered for MK-4827.[9]
PAPER
http://pubs.acs.org/doi/abs/10.1021/op400233z
Process Development of C–N Cross-Coupling and Enantioselective Biocatalytic Reactions for the Asymmetric Synthesis of Niraparib
Abstract

Process development of the synthesis of the orally active poly(ADP-ribose)polymerase inhibitor niraparib is described. Two new asymmetric routes are reported, which converge on a high-yielding, regioselective, copper-catalyzed N-arylation of an indazole derivative as the late-stage fragment coupling step. Novel transaminase-mediated dynamic kinetic resolutions of racemic aldehyde surrogates provided enantioselective syntheses of the 3-aryl-piperidine coupling partner. Conversion of the C–N cross-coupling product to the final API was achieved by deprotection and salt metathesis to isolate the desired crystalline salt form.
PAPER
1613220-15-7 cas
Free form 1038915-60-4
- 2-[4-(3S)-3-Piperidinylphenyl]-2H-indazole-7-carboxamide
- Niraparib
- Jones, Philip; Journal of Medicinal Chemistry 2009, V52(22), P7170-7185
- MK-4827
(S)-2-(4-(Piperidin-3-yl)phenyl)-2H-indazole-7-carboxamide Tosylate Monohydrate 1
Discovery of 2-{4-[(3S)-Piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide (MK-4827): A Novel Oral Poly(ADP-ribose)polymerase (PARP) Inhibitor Efficacious in BRCA-1 and -2 Mutant Tumors
Abstract

We disclose the development of a novel series of 2-phenyl-2H-indazole-7-carboxamides as poly(ADP-ribose)polymerase (PARP) 1 and 2 inhibitors. This series was optimized to improve enzyme and cellular activity, and the resulting PARP inhibitors display antiproliferation activities against BRCA-1 and BRCA-2 deficient cancer cells, with high selectivity over BRCA proficient cells. Extrahepatic oxidation by CYP450 1A1 and 1A2 was identified as a metabolic concern, and strategies to improve pharmacokinetic properties are reported. These efforts culminated in the identification of 2-{4-[(3S)-piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide 56 (MK-4827), which displays good pharmacokinetic properties and is currently in phase I clinical trials. This compound displays excellent PARP 1 and 2 inhibition with IC50 = 3.8 and 2.1 nM, respectively, and in a whole cell assay, it inhibited PARP activity with EC50 = 4 nM and inhibited proliferation of cancer cells with mutant BRCA-1 and BRCA-2 with CC50 in the 10−100 nM range. Compound 56 was well tolerated in vivo and demonstrated efficacy as a single agent in a xenograft model of BRCA-1 deficient cancer.
PATENT
https://www.google.com/patents/WO2014088983A1?cl=en

EXAMPLE 1
The following Example 1 describes synthesis of the compound 2-{4-[(3S)-Piperidin enyl}-2H-indazole-7-carboxamide:
2-{4-[(3S)-Piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide tosylate monohydrate 1
Scheme
1.1 Acylation
2- crystalline
10
A mixture of succinic anhydride 1 (110 g) and bromobenzene (695 mL) was cooled to below 5°C then added A1C13 (294 g). The slurry was allowed to warm to RT and then aged until the reaction was complete judged by HPLC. The reaction mixture was then transferred slowly into a cold HC1 solution resulting in the formation of a white precipitate. The white slurry was filtered through a fritted funnel rinsing with H20. To the off-white product was added MTBE and extracted with aq. NaOH. The aqueous layer was cooled in an ice bath. Concentrated HC1 was added drop wise to adjust the solution pH to 1 , resulting in the formation of a white slurry. The slurry was collected on a fritted funnel, rinsed with H20, and dried under vacuum with a N2 sweep at RT to give the target compound (265 g, 93% corrected yield) as a white powder.
1.2 Esterification
A mixture of the acid 2 (205 g), IPA (4 L) and cone. H2S04 (2.13 mL / 3.91 g) was heated to a gentle reflux until the reaction was complete judged by HPLC. The solution was then cooled to RT and concentrated to a volume of 350-400 mL. The residue was dissolved in
MTBE (1.2 L), washed with aq. Na2C03 followed by water. After dried over MgS04 , the filtrate was solvent-switched into heptane. The slurry was then filtered, and the cake was washed with cold heptane. After drying under vacuum, the target compound (223.5 g, 93% corrected yield) was obtained as a white powder.
1.3 Epoxidation
A mixture of Me3SOI (230 g) and DMSO (300 mL) was added KOt-Bu (113 g) followed by DMSO (300 mL). The mixture was aged for a further 1.5 hr. In a separate flask, ketone 3 (230 g) was dissolved in a mixture of THF (250 mL) and DMSO (150 mL), and the resulting solution was added drop wise to the ylide solution. The mixture was aged for 2 hr at RT, added hexanes (1 L), and then quenched by the addition of ice-water (600 mL). The layers were cut, and the organic layer was washed with water then with brine. The slightly cloudy yellow organic layer was dried over Na2S04 and filtered through a fritted funnel. Product solution assay was 176.1 g (76%> assay yield). This solution was carried forward into the rearrangement step. 1.4 Epoxide rearrangement and bisulfite formation
5 – not isolated
A solution of crude epoxide 4 (assay 59.5 g) in hexanes was solvent switched into PhMe, and added ZnBr2 (10.7 g). When the rearrangement was complete judged by HPLC, the slurry was filtered through a fritted funnel. The clear filtrate was washed with 10% aq. NaCl and then stirred with a solution of sodium bisulfite (NaHS03, 24.7 g) in H20 (140 mL) vigorously at RT for 3 hr. The cloudy aqueous layer was separated and washed with heptanes. By 1H-NMR assay, the aqueous solution contained 71.15 g bisulfite adduct 6 (30.4 wt % solution, 90%) yield from crude epoxide 4). This solution was used directly in the subsequent transaminase step.
1.5 Transaminase DKR
45 C, inert, 40-46 hrs 7
100 g/L as 17.16 wt % aq solution 99.3% ee
85-87% yield
To a cylindrical Labfors reactor was charged pyridoxal-5 -phosphate (1.4 g, 5.66 mmol), 452 ml 0.2 M borate buffer pH 10.5 containing 1M iPrNH2, 52 g transaminase (SEQ ID NO: 180), and 75 ml DMSO, and the resulting mixture was warmed to 45°C. The pH was controlled at pH 10.5 using 8 M aq iPrNH2. To this was added dropwise a mixture of 17.16 wt% aq solution of ester bi-sulfite 6 (147.2 g, 353 mmol) and 219 ml DMSO under N2 atmosphere. When the reaction was complete judged by HPLC, the reaction mixture was cooled and extracted with 1 volume of 3:2 IPA:IPAc. The aq/rag layer was extracted again with 1 volume of 3:7 IPATPAc. The organic layer was washed with brine at pH >9. Assay yield in solution was 78 g (87%); 99.3% ee. After dried over MgS04, and filtered through a fritted funnel, the crude solution was concentrated under vacuum flushing with IP Ac to remove IPA. The resulting slurry was concentrated to a final volume of -200 mL, cool to below 0°C, and filtered to collect the solid. The cake was washed with ice-cold IPAc and dried at RT under vacuum to give the desired product (84% corrected yield, 99.3 LCAP) as a white powder. 1.6. Reduction of amide
(S)-3-(4-bromophenyl)piperidine
The lactam 7 can be reduced to form the i eridine 8 as described below:
7 – crystalline
A mixture of lactam 7 (10.25 g at 97.5 wt %) in THF (100 mL) was cooled to < 10°C, and added NaBH4 (4.47 g). EtOH (6.89 mL) was then added slowly over 20 min. The slurry was aged for an additional 1 hr at 2°C after which BF3 THF (13.03 mL) was added over 1 hr. The slurry was slowly warmed to RT and aged until complete conversion judged by HPLC. The reaction was then cooled to < 5°C then slowly quenched with MeOH (7.96 mL), added HC1 (9.69 mL), then the reaction was heated to 45°C until decomplexation of product-borane complex was complete, as indicated by LC assay. The reaction was cooled, diluted with IPAc (75 mL) and water (80 mL), and then pH was adjusted with aqueous NH4OH to pH 8. The organic layer was separated, added 75 mL water, then pH adjusted to 10.5 with 50 wt % NaOH. The layers were separated and the organic layer was washed with brine. After solvent-switched to IPAc, LC Assay yield was 9.1g; 95.9%.
1.7 Tosylate salt formation The tosylate salt of the piperidine 8 can be formed as described below:
The crude piperidine 8 free base in IPA was heated to ~40°C. TsOH H20 solids was added portion-wise. The slurry was warmed to 50°C and held at that temperature for 2 h, and then slowly cooled to RT and aged overnight. Supernatant concentration was measured to be 2.5 g/ml (free base concentration). The solids were filtered and washed with IP Ac (3×15 mL) and dried at RT. Isolated solides: 14.85 g, 96% corrected isolated yield.
1.8 Boc protection
The piperi ine 8 tosylate salt can be protected as described below:
To a stirred slurry of the tosylate salt of piperidine 8 (25.03 g, 60.6 mmol) in MTBE (375 ml) was added NaOH (aq. 1.0 N, 72.7 ml, 72.7 mmol) at RT. To the mixture, (BOC)20 (13.36 ml, 57.6 mmol) was added slowly over 3 min. The resulting mixture was stirred for 4.5 hr at RT, and then the aqueous layer was separated. The MTBE layer was washed with water (100 ml X 2). The organic layer was filtered, and DMAC (100 ml) was added to the filtrate and
concentrated under vacuum. Product assay: 21.86 g, quantitative yield.
1.9 Terf-Butylamide Formation
N-(tert-butyl)- 1 H-indazole-7-carboxamide
10 11
Indazole-7-carboxylic acid 10 (50.3 g, 295 mmol) was dissolved in DMF, and added CDI (59.1 g, 354 mmol) at RT. After 1.5hr, tert-butylamine (62.5 ml, 589 mmol) was added to the reaction mixture. The resulting reaction mixture was warmed to 40 °C until complete
conversion, then cooled to RT. Water (600 ml) was added dropwise causing the mixture to form a thick slurry. Solid was collected by filtration and washed with 10% DMF in water (250 ml) followed by water. The solid was dried under vacuum. Beige solid: 55.31 g, 86%> isolated yield.
1.10 Carbon-Nitrogen Coupling
(S)-tert-butyl 3-(4-(7-(tert-butylcarbamoyl)-2H-indazol-2-yl)phenyl)piperidine- 1 -carboxylate
A mixture of the protected piperidine 9 (113 g, 18.23 wt%, 60.6 mmol) in DMAc (160 mL), compound 11 (13.82 g, 63.6 mmol), and K2CO3 (25.6 g, 182 mmol) was degassed by bubbling nitrogen. To the mixture was added CuBr (0.444 g, 3.03 mmol) and 8- hydroxyquinoline 12 (0.889 g, 6.06 mmol), and the resulting mixture was warmed to 110°C until complete conversion. The reaction mixture was then cooled, filtered through a pad of Celite, and rinsed with DMAc (100 ml). The filtrate was warmed to 35°C and added citric acid aqueous solution (10%) dropwise to form a light green slurry. After cooled to room temperature, the slurry was filtered, and the cake was washed with DMAc/Water (2/1, 150ml) followed by copious amount of water. The solid was dried under vacuum with nitrogen. Net weight: 27.24g. LC assay: 26.77g, 98.3 wt %. Assay yield: 93.6%.
1.11 Double deprotection
To compound 13 (20.0 g, 41.2 mmol) was added MSA (60 ml) and o-xylene (40 ml), and the the reaction mixture was warmed to 40°C until the complete conversion judged by HPLC. The reaction mixture was cooled to RT and added water (140 ml) slowly maintaining the temperature < 25°C. When the water addition was completed, the organic layer was removed, and the aq. layer was washed with toluene. The aqueous layer was filtered through a glass funnel, and the filtrate was added an aqueos solution of TsOH (11.77g in 23.5 ml) slowly at RT causing a thick slurry to form. Solid was collected by filtration, washed with water, and dried under vacuum. The titled compound was obtained as a white powder. Net weight: 20.6 g. LC assay: 20.0 g, 97.3 wt %. Assay yield: 95.2%.
EXAMPLE 2
The following Example 2 describes synthesis of the trifluoromethylacetate salt of compound 2-{4-[(3S)-Piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide:
2.1 Cumylamide Formation
N-(2-phenylpropan-2-yl)- 1 H-indazole-7-carboxamide
10 1 5
10
To the indazole-7-carboxylic acid 10 (400 mg, 2.47 mmol) in tetrahydrofuran (9.9 mL), was sequentially added HATU (1.13 g, 2.96 mmol), DIPEA (2.15 mL, 12.3 mmol), and cumylamine (500 mg, 3.70 mmol) at 50°C. The reaction was stirred overnight before being concentrated and loaded directly onto a silica column, eluting with 10-30% EtOAc/hexane. The product was collected and concentrated to afford the desired product as a colorless solid (557 mg, 81% yield).
2.2 Carbon-Nitrogen Coupling
-butyl 3-(4-(7-((2-phenylpropan-2-yl)carbamoyl)-2H-indazol-2-yl)phenyl)piperidine- carboxylate
15 16
A sealed vial containing the indazole-7-carboxamide 15 (50 mg, 0.18 mmol), copper(I) iodide (2.6 mg, 0.014 mmol), potassium phosphate tribasic (80 mg, 0.38 mmol), and aryl bromide 9 (73.1 mg, 0.215 mmol) was evacuated and backfilled with argon (x3). Trans-N,N’- dimethylcyclohexane-l,2-diamine (11.3 μΐ,, 0.072 mmol), and toluene (179 μΐ) were then added successively and the sealed vial was heated at 110 °C overnight. The vial was then cooled and toluene (0.30 mL) was added to the slurry. Crude LC/MS indicated >20: 1 selectivity for the desired indazole isomer. The crude product was purified by loading directly onto a Biotage Snap 10G silica column, eluting with 5-50% EtOAc/hexane. The product was collected and concentrated to afford the desired product as a colorless solid (78 mg, 81% yield).
2.3 Double deprotection
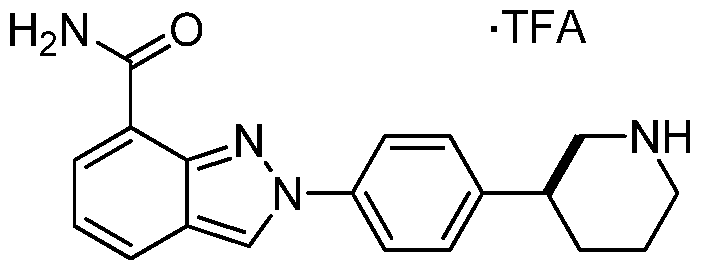
(5)-2-(4-(piperidin-3-yl)phenyl)-2H-indazole-7-carboxamide trifluoromethylacetate salt
16 17
To the piperidine-l-carboxylate 16 (45 mg, 0.084 mmol), was added triethylsilane (267 μί, 1.67 mmol) and TFA (0.965 mL, 12.5 mmol) at 25°C. The reaction was stirred for 4 hours and the reaction was concentrated in vacuo, and purified by mass triggered reverse phase HPLC (acetonitrile: water, with 0.1% TFA modifier). Lyphilization gave the desired product as the TFA salt and as a white solid (31 mg, 85% yield). HRMS (ESI) calc’d for Ci9H2iN40 [M+H]+: 321.1710, found 321.1710.
EXAMPLE 3
Following the conditions used in sections 2.1 and 2.2 of Example 2, this Example 3 shows regioselective N2 arylation of compound 9 using various amide protecting groups. The indazole-7-carboxylic acid 10 was reacted with various amines to generate a protected amide.
The amide protecting groups are indicated by the R group in Table 2. The amide coupling yield is provided in Table 2. The Cu-mediated carbon-nitrogen coupling of this indazole to compound 9 was then tested to determine if regioselective N2 arylation was possible. The arylation yield is also provided in Table 2. The data shows that various amide protecting groups on the indazole intermediate are suitable to generate efficient regioselective N2 arylation of compound 9.
| Cited Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| US8071623 * | Jan 8, 2008 | Dec 6, 2011 | Instituto Di Ricerche Di Biologia Molecolare P. Angeletti Spa | Amide substituted indazoles as poly(ADP-ribose)polymerase(PARP) inhibitors |
| US8129377 * | Sep 29, 2005 | Mar 6, 2012 | Mitsubishi Tanabe Pharma Corporation | 6-(pyridinyl)-4-pyrimidone derivates as tau protein kinase 1 inhibitors |
| US20100286203 * | Jan 8, 2009 | Nov 11, 2010 | Foley Jennifer R | Pharmaceutically acceptable salts of 2–2h-indazole-7-carboxamide |
| Reference | ||
|---|---|---|
| 1 | * | CHUNG ET AL.: “Process Development of C-N Cross-Coupling and Enantioselective Biocatalytic Reactions for the Asymmetric Synthesis of Niraparib.“, ORGANIC PROCESS RESEARCH & DEVELOPMENT, vol. 18, no. 1, 2014, pages 215 – 227, XP055263728 |
| 2 | * | JONES ET AL.: “Discovery of 2-(4-[(3S)-Piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide ( MK -4827): A Novel Oral Poly(ADP-ribose)polymerase (PARP) Inhibitor Efficacious in BRCA-1 and -2 Mutant Tumors.“, JOURNAL OF MEDICINAL CHEMISTRY, vol. 52, no. 22, 2009, pages 7170 – 7185, XP055263725 |
| 3 | * | WALLACE ET AL.: “Development of a Fit-for-Purpose Large-Scale Synthesis of an Oral PARP Inhibitor.“, ORGANIC PROCESS RESEARCH & DEVELOPMENT, vol. 15, no. 4, 2011, pages 831 – 840, XP055263721 |
| Citing Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| WO2016025359A1 * | Aug 10, 2015 | Feb 18, 2016 | Merck Sharp & Dohme Corp. | Processes for the preparation of a bace inhibitor |
References
- Jump up^ “PARP Inhibitors in Oncology. Chemosensitizers or Single-Agent Therapeutics?” (PDF). July 2009.
- Jump up^ A Phase III Trial of Niraparib Versus Physician’s Choice in HER2 Negative, Germline BRCA Mutation-positive Breast Cancer Patients (BRAVO)
- Jump up^ “PARP inhibitor, MK-4827, shows anti-tumor activity in first trial in humans”. 17 Nov 2010.
- ^ Jump up to:a b c Tesaro’s PARP ovarian cancer drug hits PhIII goal; prepares to file. June 2016
- Jump up^ MK-4827 @ pubchem
- Jump up^ MK-4827
- Jump up^ Murai, Junko, et al. “Trapping of PARP1 and PARP2 by clinical PARP inhibitors.” Cancer research 72.21 (2012): 5588-5599.
- Jump up^ AbbVie takes PARP inhibitor into third phase III trial. June 2014
- Jump up^ Niraparib studies
Further reading
 |
|
| Clinical data | |
|---|---|
| Routes of administration |
By mouth |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | 1038915-60-4  |
| PubChem (CID) | 24958200 |
| ChemSpider | 24531930  |
| UNII | HMC2H89N35  |
| ChEMBL | CHEMBL1094636  |
| Chemical and physical data | |
| Formula | C19H20N4O |
| Molar mass | 320.394 g/mol |
| 3D model (Jmol) | Interactive image |
Filed under: Uncategorized Tagged: MK 4827, Niraparib