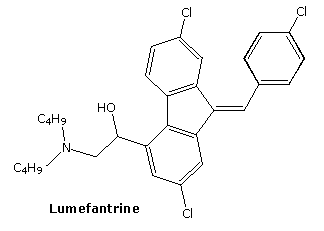
lumefantrine
2-(dibutylamino)-1-[(9Z)-2,7-dichloro-9-[(4-chlorophenyl)methylidene]-9H-fluoren-4-yl]ethan-1-ol
| (±)-2,7-Dichloro-9-((Z)-p-chlorobenzylidene)-α-((dibutylamino)methyl)fluorene-4-methanol |
| 2-Dibutylamino-1-[2,7-dichloro-9-(4-chloro-benzylidene)-9H-fluoren-4-yl]-ethanol |
| 2-Dibutylamino-1-{2,7-dichloro-9-[1-(4-chloro-phenyl)-meth-(Z)-ylidene]-9H-fluoren-4-yl}-ethanol |
| Benflumetol |
| dl-Benflumelol |
UNII F38R0JR742
CAS number 82186-77-4
Weight Average: 528.94
Monoisotopic: 527.154947772
Chemical Formula C30H32Cl3NO
Lumefantrine (or benflumetol) is an antimalarial drug. It is only used in combination with artemether. The term “co-artemether” is sometimes used to describe this combination.[1] Lumefantrine has a much longer half-life compared to artemether and so is therefore thought to clear any residual parasites that remain after combination treatment.[2]
Lumefantrine, along with pyronaridine and naphtoquine, were synthesized in course of the Project 523 antimalaria drug research initiated in 1967; these compounds are all used in combination antimalaria therapies.[3][4][5]

Lumefantrine is an antimalarial drug chemically known as 2-(dibutylamino)-1-[(9Z)-2, 7-dichloro-9-(4- chlorobenzylidene)-9H-floren-4-yl] ethanol, which is used in the prevention and treatment of Malaria in worm blooded animals. Lumefantrine is using the combination of β-Artemether in the treatment of Malaria
http://derpharmachemica.com/vol8-iss3/DPC-2016-8-3-91-100.pdf

REFERENCES
[1] Ulrich Beutler, C Peter.; Fuenfschilling.; and Andreas, Steinkemper.; Novartis Pharma AG; Chemical and Analytical Development: CH-4002 Basel, Switzerland, Organic Process Research & Development 2007, 11, 341- 345.
[2] Boehm, M. Fuenfschilling.; Krieger, P. C.; Kuesters, E. M.; Struber, F.; Org. Process Res. DeV. 2007, 11, 336- 340.
[3] (a) Rao, D. R.; Kankan, R. N.; Phull, M. S.; Patent Application CN 1009-3724 20060424, 2005. (b) Deng, R.; Zhong, J.; Zhao, D.; Wang, J.; Yaoxue, X. 2000, 35 (1), 22. (c) Allmendinger, Th.; Wernsdorfer, W. H. PCT WO 99/67197.
[4] Perrumattam, J.; Shao, Ch.; Confer, W. L. Synthesis 1994, 1181.
[5] Fuenfschilling, P. C.; Hoehn P.; Mutz J.-P. Organic Process Res. Dev. 2007, 11, 13.
[6] Di Nunno, L.; Scilimati, A. Tetrahedron 1988, 44, 3639.
[7] Pharmacopeial Forum, Vol. 36(2) [Mar.-Apr. 2010]
Preparation of 2-(dibutylamino)-1-[(9Z)-2, 7-dichloro-9-(4-chlorobenzylidene)-9H-floren-4-yl] ethanol (Lumefantrine) 1.
To a stirred solution of NaOH (1.97 g 0.0492 mol) in methanol (100 ml) there was added 1-(2, 7- dichloro-9 H-fluren-4-yl)-2-(dibutyl amino) ethanol (10 g, 0.0246 mol) and para chloro benzaldehyde (5.24 g 0.0372). The suspension obtained was stirred at reflux temperature till the absence of starting material by TLC. After confirming the product formation reaction mixture was cooled to room temperature and further stirred at same temperature for overnight. The precipitated solids were filtered and washed with methanol and dried under vacuum at 50°C to get desired compound. (Purity by HPLC: 99%).
IR (cm-1): 3408, 3092, 2953, 2928, 2870, 2840, 1634, 1589, 1487, 1465, 1443, 1400, 1365, 1308, 1268, 1241, 1207, 1173, 1156, 1085, 1071, 1014, 980, 933, 874, 839, 815, 806, 770;
1H NMR (CDCl3, δ ppm): 7.75 (d, 1H, CH, J 1.5 Hz), 7.68 (d, 1H, CH, J 1.5 Hz), 7.60-7.63 (m, 1H, CH), 7.32-7.35 (dd, 1H, CH, J 1.7,8.3 Hz), 7.45-7.50 (m, 1H, CH), 5.35-5.39 (dd, 1H, CH, J 3.0,9.9 Hz), 2.41-2.74 (m, 1H, CH2Ha), 2.86-2.92 (m, 1H, CH2Hb), 2.41-2.74 (m, 4H, CH2), 1.25-1.56 (m, 8H, CH2), 0.97 (t, 1H, CH, J 7.2 Hz), 7.60-7.63 (m, 1H, CH), 7.45-7.50 (m, 4H, CH), 4.54 (broad, 1H, OH),
13C NMR (CDCl3, δ ppm): 138.2, 141.5, 120.6, 133.2, 126.3, 135.0, 135.0, 136.4, 123.9, 128.3, 132.8, 123.0, 139.8, 65.5, 60.0, 53.5, 29.1, 20.6, 14.0, 127.6, 134.7, 130.5, 129.1, 133.2;
MS: m/z: 528 [M+H]+ ; Analysis calcd. for C30H32Cl3NO: C, 68.12; H, 6.10; N, 2.65% Found: C, 68.38; H, 6.14; N, 2.63 %.
CLIP
One-dimensional 1H NMR spectrum of B) a lumefantrine standard,
A CLIP
http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-50532012000100010&lng=en&nrm=iso




CLIP
A simple and precise method for quantitative analysis of lumefantrine …
[2–4] Thus, today lumefantrine is a drug of choice in antimalarial treatment against P. …. The NMRspectra observed triplet at 0.943-0.989 (methyl protons of alkyl …







References
- Jump up^ Toovey S, Jamieson A, Nettleton G (2003). “Successful co-artemether (artemether-lumefantrine) clearance of falciparum malaria in a patient with severe cholera in Mozambique”. Travel medicine and infectious disease. 1 (3): 177–9. doi:10.1016/j.tmaid.2003.09.002. PMID 17291911.
- Jump up^ White, Nicholas J.; van Vugt, Michele; Ezzet, Farkad (1999). “Clinical Pharmacokinetics and Pharmacodynamics of Artemether-Lumefantrine”. Clinical Pharmacokinetics. 37 (2): 105–125. doi:10.2165/00003088-199937020-00002. ISSN 0312-5963.
- Jump up^ Cui, Liwang; Su, Xin-zhuan (2009). “Discovery, mechanisms of action and combination therapy of artemisinin”. Expert Review of Anti-infective Therapy. 7 (8): 999–1013. doi:10.1586/eri.09.68. PMC 2778258
![Freely accessible Freely accessible]() . PMID 19803708.
. PMID 19803708. - Jump up^ http://aac.asm.org/content/56/5/2465.full
- Jump up^ Laman, M; Moore, BR; Benjamin, JM; Yadi, G; Bona, C; Warrel, J; Kattenberg, JH; Koleala, T; Manning, L; Kasian, B; Robinson, LJ; Sambale, N; Lorry, L; Karl, S; Davis, WA; Rosanas-Urgell, A; Mueller, I; Siba, PM; Betuela, I; Davis, TM (2014). “Artemisinin-naphthoquine versus artemether-lumefantrine for uncomplicated malaria in Papua New Guinean children: an open-label randomized trial”. PLoS Med. 11: e1001773. doi:10.1371/journal.pmed.1001773. PMC 4280121
![Freely accessible Freely accessible]() . PMID 25549086.
. PMID 25549086.
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a609024 |
| Routes of administration |
Oral |
| ATC code | P01BF01 (WHO) (combination with artemether) |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | 82186-77-4  |
| PubChem (CID) | 6437380 |
| DrugBank | DB06708  |
| ChemSpider | 4941944  |
| UNII | F38R0JR742  |
| KEGG | D03821  |
| ChEBI | CHEBI:156095  |
| ChEMBL | CHEMBL38827  |
| Chemical and physical data | |
| Formula | C30H32Cl3NO |
| Molar mass | 528.939 g/mol |
| 3D model (Jmol) | Interactive image |
///////////lumefantrine
CCCCN(CCCC)CC(O)C1=C2C(=CC(Cl)=C1)\C(=C/C1=CC=C(Cl)C=C1)C1=C2C=CC(Cl)=C1
Filed under: Uncategorized Tagged: lumefantrine

