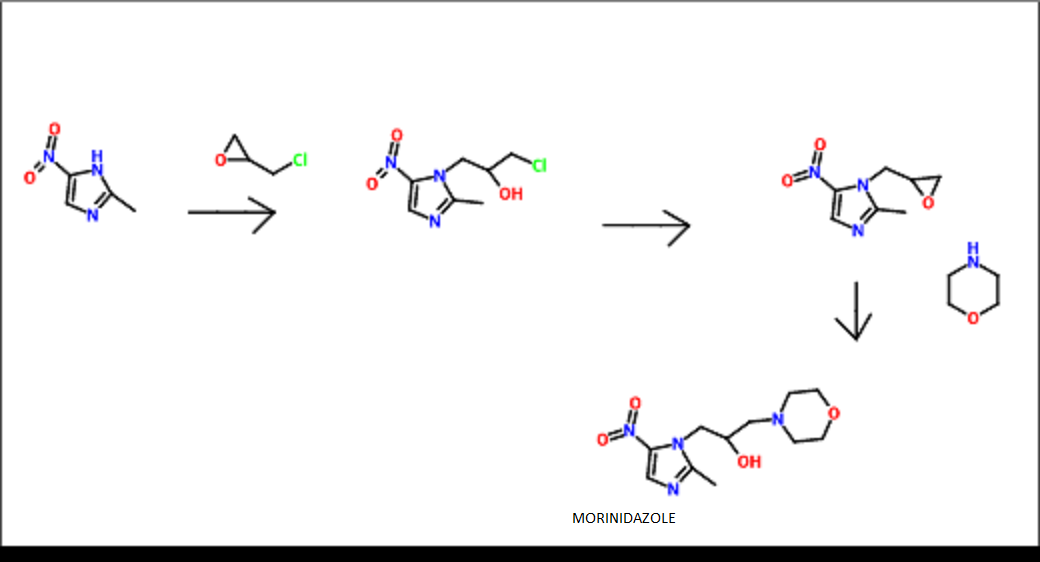
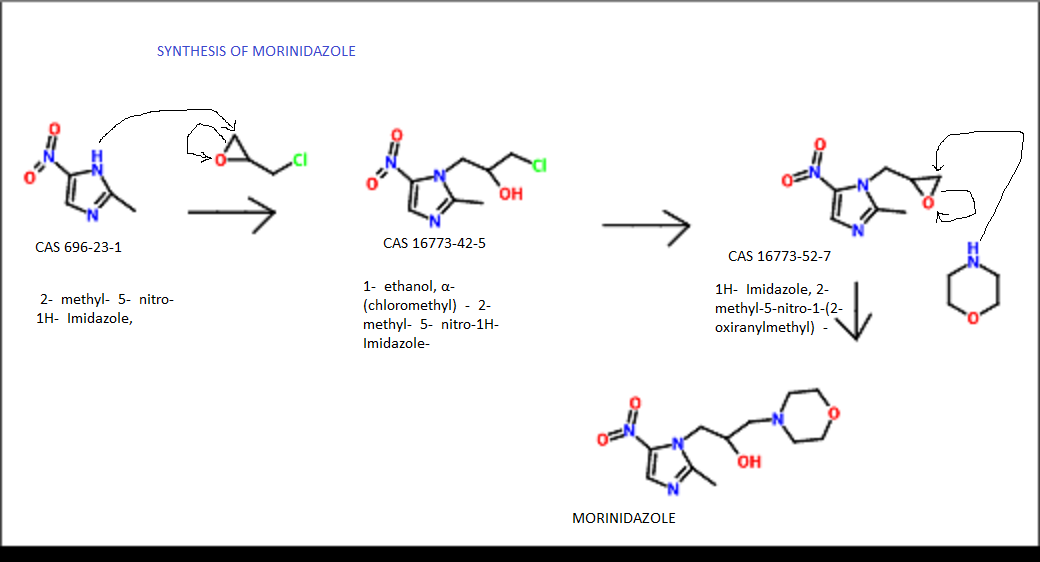
MORINIDAZOLE
1- [3- (4-morpholinyl) -2-hydroxypropyl] -2-methyl-5- nitro -1H- imidazole
CAS 92478-27-8
Jiangsu Hansoh Pharmaceutical Co., Ltd.
Morinidazole was approved by China Food and Drug Administration (CFDA) on February 24, 2014. It was developed and marketed as a step Lingda ® by Hansoh Pharmaceutical.
Morinidazole is a nitroimidazoles antibiotic indicated for the treatment of bacterial infections including appendicitis and pelvic inflammatory disease (PID) caused by anaerobic bacteria.
PATENT
WO2006058457A1.
http://www.google.com/patents/WO2006058457A1?cl=en
……………………….
PATENT
CN1981764A.
https://www.google.com/patents/CN1981764A?cl=en
1- (2,3-epoxypropoxy yl) -2-methyl-5-nitro-imidazole (10g), morpholino (10g), 100ml of acetonitrile under reflux for 2 hours, vacuum recovery of acetonitrile, water was added 100ml, heating to the whole solution, filtered hot, let cool, filtering, washing and drying to obtain an off-white solid (11g).
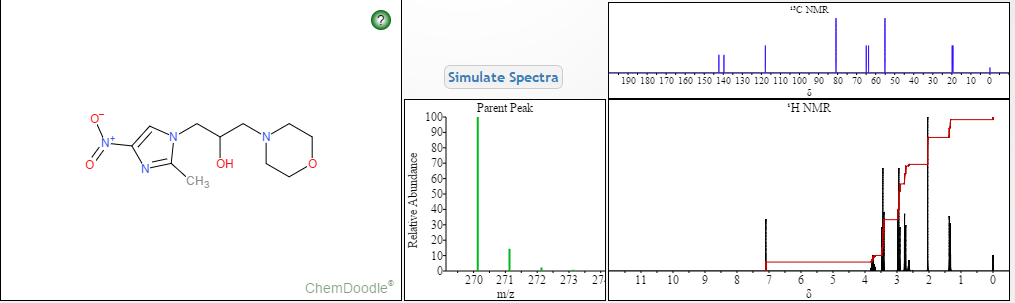
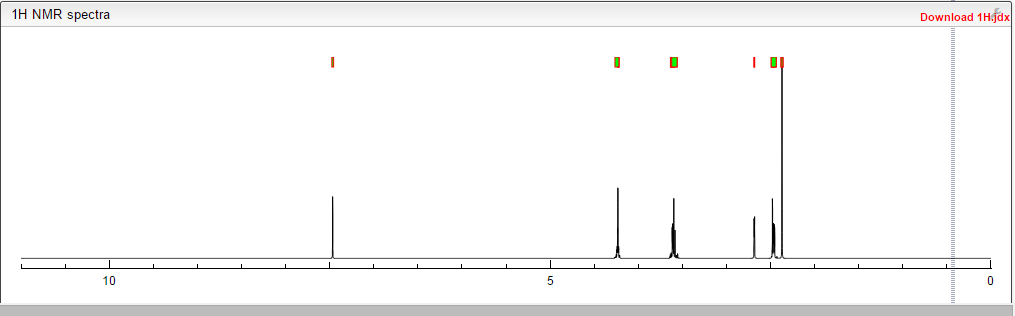
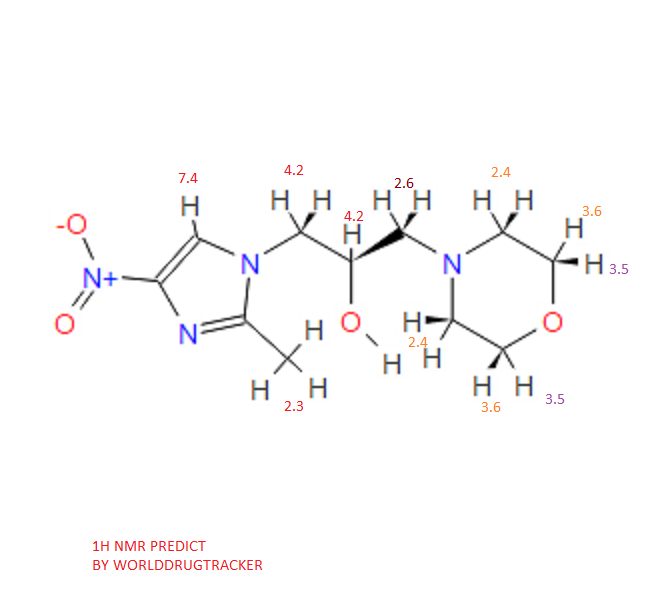
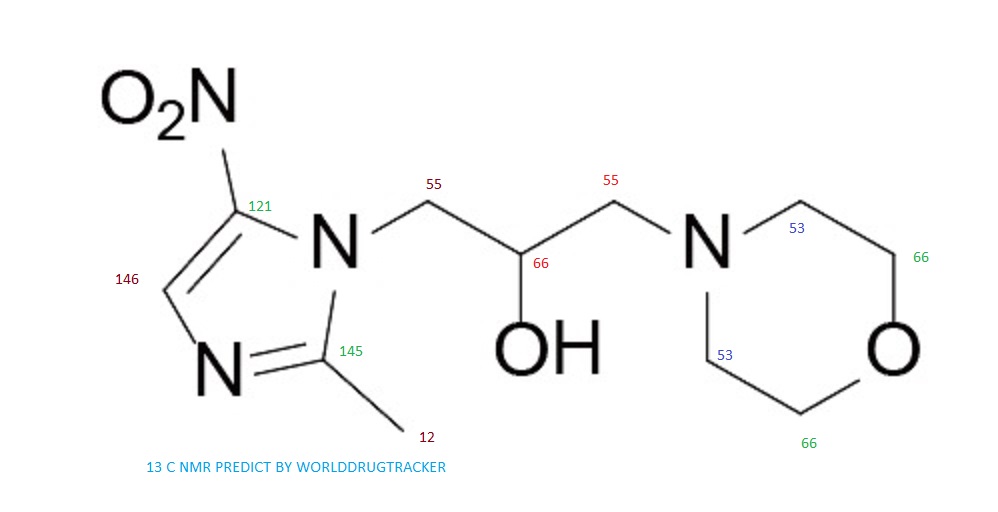
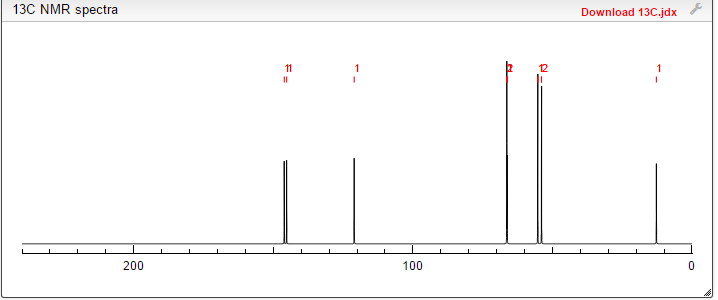
Proton nuclear magnetic resonance data: 1HNMR (CD3Cl) δ2.39 ~ 2.73 (6H, m) δ2.61 (3H, s) δ3.71 ~ 3.81 (4H, m) δ4.10 ~ 4.17 (2H, m) δ4 .63 ~ 4.66 (1H, m) δ8.00 (1H, s)
CN 102199147
http://www.google.com/patents/CN102199147A?cl=en
CN 1605586
https://www.google.com/patents/CN1605586A?cl=en
Example 7 Preparation of α- (morpholino-1-yl) methyl-2-methyl-5-nitroimidazole-1-ethanol according to Example 4 the same manner as in Preparation α- (morpholino-1-yl) methyl-2-methyl-5-nitroimidazole-1-ethanol, except for using morpholine instead of 4-hydroxypiperidine, prepared by the present invention Compound 7. Proton nuclear magnetic resonance data: 1HNMR (CD3Cl) δ2.39 ~ 2.73 (6H, m) δ2.61 (3H, s) δ3.71 ~ 3.81 (4H, m) δ4.10 ~ 4.17 (2H, m) δ4
NMR PREDICT
1H NMR PREDICT
13C NMR PREDICT
COSY
| CN1810815B | Mar 8, 2006 | Mar 16, 2011 | 陕西合成药业有限公司 | Nitroimidazole derivative for treatment |
| CN1903846B | Aug 15, 2006 | Jul 13, 2011 | 杨成 | Ornidazole derivative used for therapy, its preparation method and use |
| CN100387233C | Jun 9, 2006 | May 14, 2008 | 南京圣和药业有限公司 | Use of levo morpholine nidazole for preparing medicine for antiparasitic infection |
| CN100427094C | Dec 13, 2005 | Oct 22, 2008 | 江苏豪森药业股份有限公司 | Usage of alpha-(Morpholin-1-base) methyl-2-methyl-5-azathio-1-alcohol in preparation of anti-trichomoniasis and anti-ameba medicines |
| CN100540549C | Dec 15, 2005 | Sep 16, 2009 | 南京圣和药业有限公司 | Alpha-substituted-2-methyl-5-nitro-diazole-1-alcohol derivative with optical activity |
| WO2007079653A1 * | Dec 25, 2006 | Jul 19, 2007 | Junda Cen | OPTICALLY PURE α-SUBSTITUTED 2-METHYL-5-NITROIMIDAZOLE-1-ETHANOL DERIVATIVES |

Filed under: cfda, china pipeline, Uncategorized Tagged: China Food and Drug Administration, Jiangsu Hansoh Pharmaceutical, MORINIDAZOLE, pelvic inflammatory disease