 Pirarubicin Hydrochloride
Pirarubicin Hydrochloride 
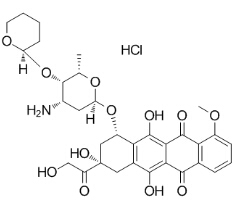
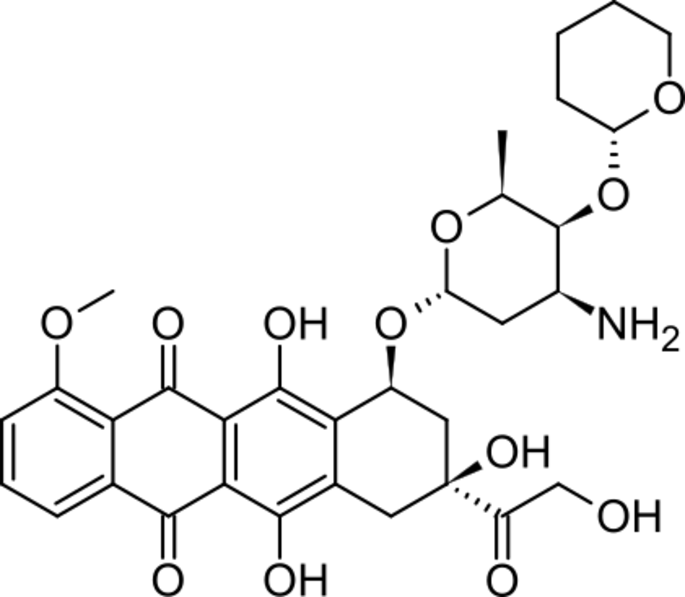
(7S,9S)-7-((2R,4S,5S,6S)-4-amino-6-methyl-5-((R)-tetrahydro-2H-pyran-2-yloxy)-tetrahydro-2H-pyran-2-yloxy)-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione HCl
THP Hydrochloride
(7S,9S)-7-((2R,4S,5S,6S)-4-amino-6-methyl-5-((R)-tetrahydro-2H-pyran-2-yloxy)-tetrahydro-2H-pyran-2-yloxy)-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione HCl
MF C32H38ClNO12
MW 664.1
BASE 72496-41-4
Pirarubicin
or Pinorubicin
or Therarubicin
or (8S,10S)-10-(((2R,4S,5S,6S)-4-Amino-6-methyl-5-(((R)-tetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H-pyran-2-yl)oxy)-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione
or Pirarubicin
Pirarubicin Hcl is an analogue of the anthracycline anti-neoplastic doxorubicin, which is an inhibitor of Topo II.
Target: Topoisomerase
Pirarubicin is an anthracycline drug. An analogue of the anthracycline antineoplastic antibiotic doxorubicin. Pirarubicin intercalates into DNA and interacts with topoisomerase II, thereby inhibiting DNA replication and repair and RNA and protein synthesis. This agent is less cardiotoxic than doxorubicin and exhibits activity against some doxorubicin-resistant cell lines.
Pirarubicin (THP-adriamycin or THP-doxorubicin) was found during a search of new anthracycline antibiotics among 4′-O-substituted compounds having less toxicities than other anthracycline anticancer drugs in 1979 by Umezawa et al. In its preclinical studies, this compound possessed almost similar antitumor efficacies to doxorubicin, but was effective against doxorubicin-resistant P388 and other murine tumor cell lines. This compound was rapidly incorporated into tumor cells, inhibiting DNA polymerase alpha and subsequently DNA synthesis.
Inhibition of RNA synthesis was also noted. In the clinical studies, clinical responses were established against head and neck cancer, breast cancer, urogenital cancers, ovarian cancer, uterine cancer, acute leukemia, and malignant lymphoma, showing a wide antitumor spectrum clinically. Among the side effects, cardiac toxicity, alopecia and disturbance of the digestive organs were mild. From these results, THP-adriamycin seems to be a useful clinical drug for human solid tumors.
Pirarubicin (INN) is an anthracycline drug. An analogue of the anthracycline antineoplastic antibiotic doxorubicin. Pirarubicin intercalates into DNA and interacts with topoisomerase II, thereby inhibiting DNA replication and repair and RNA and protein synthesis. This agent is less cardiotoxic than doxorubicin and exhibits activity against some doxorubicin-resistant cell lines
 .
.
EP 0014853
https://www.google.com/patents/EP0014853B1?cl=en

 |
|
| Systematic (IUPAC) name | |
|---|---|
| (3S)-3-glycoloyl-3,5,12-trihydroxy-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-4-O-[(2R)-tetrahydro-2H-pyran-2-yl]-α-L–lyxo-hexopyranoside | |
| Clinical data | |
| AHFS/Drugs.com | International Drug Names |
|
|
| Identifiers | |
72496-41-4  |
|
| L01DB08 | |
| PubChem | CID 3033521 |
| ChemSpider | 2298189  |
| UNII | D58G680W0G  |
| KEGG | D01885  |
| ChEMBL | CHEMBL1398373  |
| Synonyms | (9S)-7-[(2R,4S,5S,6S)-4-amino-6-methyl-5-[(2R)-oxan-2-yl]oxyoxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione |
| Chemical data | |
| Formula | C32H37NO12 |
| 627.63 g/mol | |
TAKE A TOUR
Bijapur, Karnataka, INDIA
-
Bijapur – Wikipedia, the free encyclopedia
en.wikipedia.org/wiki/BijapurVijayapur city, formerly Bijapur, is the district headquarters of Bijapur District of Karnataka state. It is also the headquarters for Bijapur Taluka. Bijapur city is well …
.
 .
.






 GOLCONDA
GOLCONDA


Badami Cave Temple, near Bijapur





//////////
Filed under: Uncategorized Tagged: Bijapur, INDIA, KARNATAKA, Pinorubicin, Pirarubicin, Pirarubicin Hydrochloride, Therarubicin
