
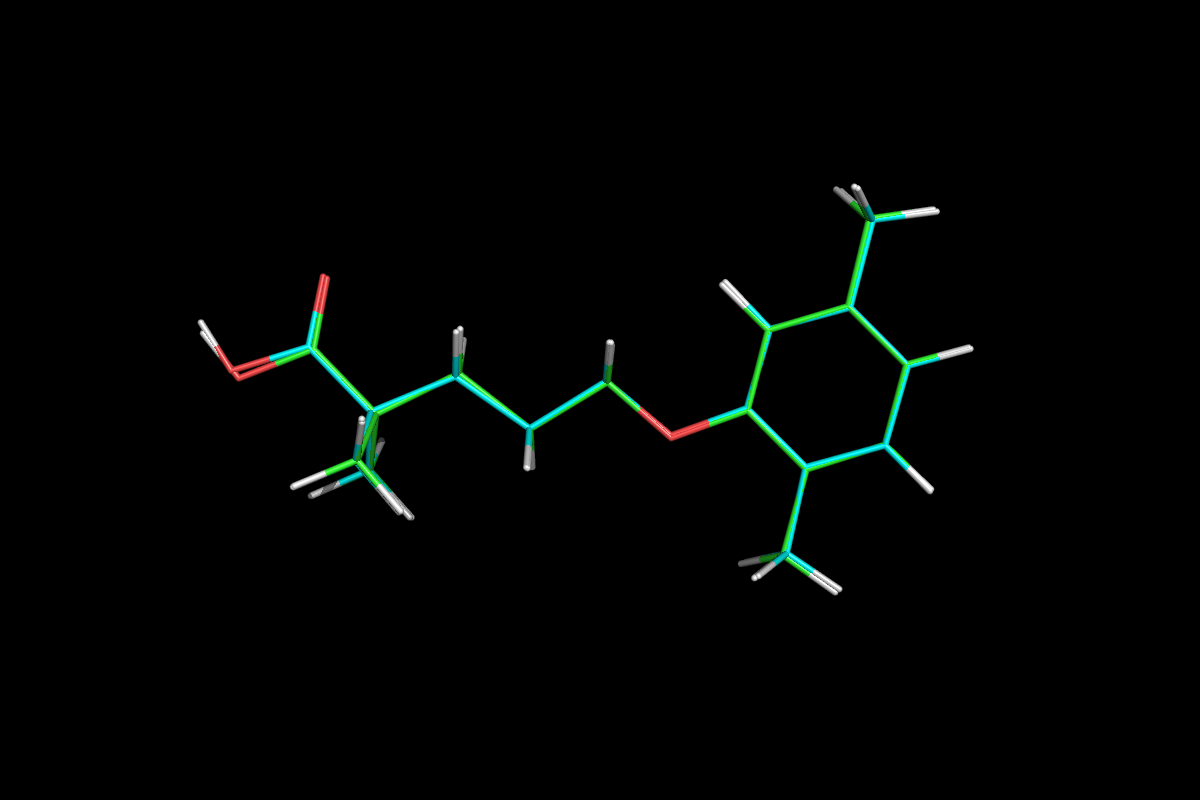
Gemfibrozil
5-(2,5-Dimethylphenoxy)-2,2-dimethylpentanoic Acid
IR (KBr, cm–1): 2959.03, 2919.78, 2877.65, 1709.42, 1613.44, 1586.60, 1511.07, 1473.81, 1414.01, 1387.89, 1317.61, 1286.34, 1271.91, 1214.39, 1159.26, 1048.83, 996.57, 803.75;
1H NMR (DMSO, 500 MHz, δ ppm): 1.12 (s, 6H), 1.60 and 1.67 (m, 4H), 2.08 (s, 3H), 2.24 (s, 3H), 3.90 (t, 2H), 6.62 (d, 1H), 6.70 (s, 1H), 6.97 (d, 1H);
13C NMR and DEPT (DMSO, 500 MHz, δ ppm): 15.39 (CH3), 20.94 (CH3), 24.67 (CH2), 24.87 (CH3, CH3), 36.43 (CH2), 40.91 (C), 67.57 (CH2), 112.07 (CH), 120.45 (CH), 122.44 (C), 129.96 (CH), 135.93 (C), 156.43 (C), 178.56 (C);
MS M/Z (ESI): 251.16 [(MH)+].
Solvent:CDCl3Instrument Type:JEOLNucleus:1HFrequency:400 MHzChemical Shift Reference:TMS
1H NMR spectrum of C15H22O3 in CDCL3 at 400 MHz
Gemfibrozil is the generic name for an oral drug used to lower lipid levels. It belongs to a group of drugs known as fibrates. It is most commonly sold as the brand name, Lopid. Other brand names include Jezil and Gen-Fibro.
history
Gemfibrozil was selected from a series of related compounds synthesized in the laboratories of the American company Parke Davisin the late 1970s. It came from research for compounds that lower plasma lipid levels in humans and in animals.[1]
Actions
- Is an activator of peroxisome proliferator-activated receptor-alpha (PPARα), a nuclear receptor that is involved in metabolism ofcarbohydrates and fats, as well as adipose tissue differentiation. This increase in the synthesis of lipoprotein lipase thereby increases the clearance of triglycerides.[citation needed]
Therapeutic effects
- Reduce triglyceride levels [2]
- Reduce very low density lipoprotein (VLDL) levels
- Modest reduction of low density lipoprotein (LDL) levels
- Moderate increase in high density lipoprotein (HDL) levels
Nontherapeutic effects and toxicities
- GI distress
- Musculoskeletal pain
- Increased incidence of gallstone
- Hypokalemia (low blood potassium)
- Increased risk of cancer
Indications
- Hyperlipidemia (Type III): Gemfibrozil is the drug of choice for therapy.
- Hypertriglyceridemia (Type IV): Gemfibrozil, though not as effective as niacin, is better tolerated.[citation needed]
Contraindications and precautions
- Gemfibrozil should not be given to these patients:
- Hepatic dysfunction
- Gemfibrozil should be used with caution in these higher risk categories:
- Biliary tract disease
- Renal dysfunction
- Pregnant women
- Obese patients
Drug interactions
- Anticoagulants: Gemfibrozil potentiates the action of warfarin and indanedione anticoagulants.[citation needed]
- Statin drugs: Concomitant administration of fibrates (including gemfibrozil) with statin drugs increases the risk of muscle cramping, myopathy, andrhabdomyolysis.
- Gemfibrozil inhibits the activation of the liver’s Cytochrome P450 system, reducing hepatic metabolism of many drugs, and prolonging their half lives and duration of action.
- Drugs metabolized by the Cytochrome P450 system include:
- Many antidepressants
- Many antipsychotics
- Many antiepileptics
- Theophylline and other methylxanthine drugs
- Several anesthetic agents
- Oral contraceptive pills
- Statins
- Warfarin
- Drugs metabolized by the Cytochrome P450 system include:
Environmental data
Gemfibrozil has been detected in biosolids (the solids remaining after wastewater treatment) at concentrations up to 2650 ng/g wet weight.[3] This indicates that it survives the wastewater treatment process.
SYNTHESIS


The sodium isobutyrate (I) is metallated with lithium diisopropylamide, and the resulting compound is alkylated with 3- (2,5-dimethylphenoxy) propyl bromide.
PATENT
Paul, L. C. 2,2-Dimethyl-ω-aryloxy alkanoic acids and salts and ester thereof. U.S. 3,674,836, 1972.
http://www.google.co.in/patents/US3674836
CLIP
Production of Gemfibrozil
(1)2,5-Dimethylphenol and 1-Bromo-3-chloropropane reaction of 1-(2,5-dimethylphenoxy)-3-chloropropane. The reaction is carried out in toluene, adding new clean off reflux 5h. Just as follows:

(2)N/A can be used to manufacture Gemfibrozil.

PAPER
Improved Process for Preparation of Gemfibrozil, an Antihypolipidemic
An improved process for the preparation of gemfibrozil, an antihypolipodimic drug substance, with an overall yield of 80% and ∼99.9% purity (including three chemical reactions) is reported. Formation and control of possible impurities are also described. Finally, gemfibrozil is isolated from water without any additional solvent purification.
Literature References:
Serum lipid regulating agent. Prepn: P. L. Creger, DE 1925423; eidem, US 3674836 (1969, 1972, both to Parke, Davis).
Production: O. P. Goel, US 4126637 (1978 to Warner-Lambert).
Pharmacology: A. H. Kissebach et al.,Atherosclerosis 24, 199 (1976); M. T. Kahonen et al., ibid. 32, 47 (1979).
Series of articles on metabolism, clinical pharmacology, kinetics and toxicology: Proc. R. Soc. Med. 69, Suppl 2, 1-120 (1976).
Toxicity data: S. M. Kurtz et al., ibid. 15.
Clinical trial in hyperlipidemia: J. E. Lewis et al., Pract. Cardiol. 9, 99 (1983).
Clinical reduction of cardiovascular risk in patients with low HDL levels: H. B. Rubins et al., N. Engl. J. Med. 341, 410 (1999).
References
- Rodney, G; et al. (1976). “The Hypolipidemic Effect of Gemfibrozil (CI-719) in Laboratory Animals”. Proc. roy. Soc. Med. 69 (Supplement 2): 6–9. PMC 1864017
![free to read free to read]() . PMID 828263.
. PMID 828263. - “Gemfibrozil.” WebMD.com Accessed 14 June 2014. http://www.webmd.com/drugs/drug-11423-gemfibrozil+oral.aspx
- http://water.epa.gov/scitech/wastetech/biosolids/tnsss-overview.cfm
External links
- DrugBank Gemfibrozil
- NIH Gemfibrozil Drug Info
- Lopid International Study
- Author (2004). “Safety of Statins”. Circulation. 109: III–50–III–57. doi:10.1161/01.cir.0000131519.15067.1f.
- http://www.ihs.gov/nptc/documents/NPTC%20Lipid%20Review.pdf
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
5-(2,5-dimethylphenoxy)-2,2-dimethyl-pentanoic acid
|
|
| Clinical data | |
| Trade names | Lopid |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a686002 |
| Pregnancy category |
|
| Routes of administration |
Oral |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Close to 100% |
| Protein binding | 95% |
| Metabolism | Hepatic (CYP3A4) |
| Biological half-life | 1.5 hours |
| Excretion | Renal 94% Feces 6% |
| Identifiers | |
| CAS Number | 25812-30-0  |
| ATC code | C10AB04 (WHO) |
| PubChem | CID 3463 |
| IUPHAR/BPS | 3439 |
| DrugBank | DB01241  |
| ChemSpider | 3345  |
| UNII | Q8X02027X3  |
| KEGG | D00334  |
| ChEBI | CHEBI:5296  |
| ChEMBL | CHEMBL457  |
| Chemical data | |
| Formula | C15H22O3 |
| Molar mass | 250.333 g/mol |
LOPID® (gemfibrozil tablets, USP) is a lipid regulating agent. It is available as tablets for oral administration. Each tablet contains 600 mg gemfibrozil. Each tablet also contains calcium stearate, NF; candelilla wax, FCC; microcrystalline cellulose, NF; hydroxypropyl cellulose, NF; hypromellose, USP; methylparaben, NF; Opaspray white; polyethylene glycol, NF; polysorbate 80, NF; propylparaben, NF; colloidal silicon dioxide, NF; pregelatinized starch, NF. The chemical name is 5-(2,5-dimethylphenoxy)2,2-dimethylpentanoic acid, with the following structural formula:
 |
The empirical formula is C15H22O3 and the molecular weight is 250.35; the solubility in water and acid is 0.0019% and in dilute base it is greater than 1%. The melting point is 58° –61°C. Gemfibrozil is a white solid which is stable under ordinary conditions.
/////////Gemfibrozil, Antilipemic, Fibrates, 25812-30-0,
CC1=CC(OCCCC(C)(C)C(O)=O)=C(C)C=C1
Filed under: Uncategorized Tagged: 25812-30-0, Antilipemic, Fibrates, Gemfibrozil



 .
.