DRUG REGULATORY AFFAIRS INTERNATIONAL

This additional specification describes the way files should be constructed for inclusion in the eCTD.
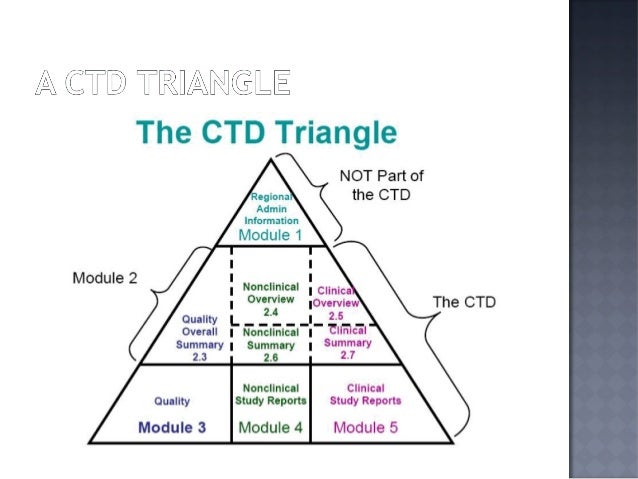 Key Points:
Key Points:
- It is not necessary to use a product from Adobe or from any specific company to produce PDF documents.
- All ICH regional regulatory authorities are able to read and accept PDF files saved as PDF version 1.4 through 1.7, PDF/A-1, or PDF/A-2 compliant to ISO 32000-1:2008.
- The size of a PDF file should not exceed 500MB.
- Regulatory authorities cannot guarantee the availability of any fonts except Times New Roman, Arial, and Courier and fonts supported in the Acrobat product set itself. Therefore, all additional fonts used in the PDF files should be embedded to ensure that those fonts would always be available to the reviewer.
- Times New Roman, 12-point font, is adequate in size for narrative text and should be used whenever possible. Times New Roman font sizes 9-10 or an equivalent size…
View original post 493 more words
Filed under: Uncategorized