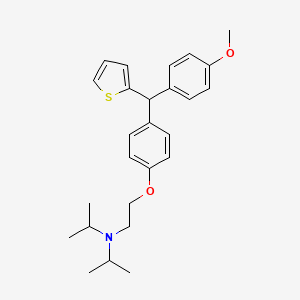
CDRI 830
CDRI S006-830
N-[2-[4-[(4-methoxyphenyl)-thiophen-2-ylmethyl]phenoxy]ethyl]-N-propan-2-ylpropan-2-amine
| Molecular Formula: | C26H33NO2S |
|---|---|
| Molecular Weight: | 423.61072 g/mol |

CDRI-830 of thiophene containing trisubstituted methane (TRSM) class was identified as an anti-tubercular lead with MIC value of 1.33 mg/L against Mycobacterium tuberculosis H37Rv strain, non-toxicity against Vero C-1008 cell line (selectivity index >10), ex vivo efficacy (in mouse and human macrophages) equivalent to first line TB drugs, lung CFU count (2.2×107) comparable to pyrazinamide (1.9×107) and ethambutol (1.27×107). CDRI-830 has exhibited potent bactericidal activity against single and multi-drug resistant clinical isolates of M. tuberculosis. Furthermore, CDRI-830 has demonstrated good pharmacokinetic properties with fast intestinal absorption, peak plasma concentration one hour post oral dose, optimum elimination half-life (9-13 h), plasma protein binding (~60%), favorable bioavailability (45-50%) and mean residence time (18-20 h).

CDRI S006-830 is a potent triethylamine containing thiophene antitubercular compound of the Central Drug Research Institute, India. The present study aimed to conduct comprehensive metabolic investigations of CDRI S006-830 to corroborate its preclinical investigations. Preliminary metabolic investigations were performed to assess the metabolic stability, enzyme kinetics, reaction phenotyping, and metabolite identification of CDRI S006-830 in rat, rabbit, dog, and human liver microsomes using liquid chromatography with mass spectrometry. The observed in vitro t1/2 and Clint values were 9.9 ± 1.29, 4.5 ± 0.52, 4.5 ± 0.86, 17 ± 5.21 min and 69.60 ± 8.37, 152.0 ± 17.26, 152.34 ± 27.63, 33.62 ± 21.04 μL/min/mg in rat, rabbit, dog and human liver microsomes respectively. These observations suggested that CDRI S006-830 rapidly metabolized in the presence of NADPH in liver microsomes of rat, rabbit and dog while moderately metabolized in human liver microsomes. It was observed that CDRI S006-830 exhibited monophasic Michaelis–Menten kinetics. The metabolism of CDRI S006-830 was primarily mediated by CYP3A4 and was deduced by CYP reaction phenotyping with known potent inhibitors. CYP3A4 involvement was also confirmed by cDNA-expressed recombinant human isozyme activity with different CYPs. Four major phase-I metabolites of S006-830, (M-1 to M-4) were detected in rat, rabbit, dog (except M4) and human liver microsomes……..http://onlinelibrary.wiley.com/doi/10.1002/dta.1802/abstract?systemMessage=Wiley+Online+Library+will+be+unavailable+on+Saturday+14th+May+11%3A00-14%3A00+BST+%2F+06%3A00-09%3A00+EDT+%2F+18%3A00-21%3A00+SGT+for+essential+maintenance.Apologies+for+the+inconvenience.
13C NMR

Abstract


Total Synthesis of an Experimental Antitubercular DrugDOI:
10.1080/00397911.2014.942745
Uma Reddy Paillaab, Veera Reddy Aravaa* & L. K. Ravindranathb
pages 3408-3413
http://www.tandfonline.com/doi/abs/10.1080/00397911.2014.942745
REFERENCES
http://www.ingentaconnect.com/content/ben/cpa/2015/00000011/00000001/art00008?crawler=true
S006-830 against H37RV, single, multi-drug resistant M. tuberculosis; CFU in the lungs with S006-830, EMB, PZA (European Journal of Medicinal Chemistry 2015, 95, 357-368, J Antimicrob Chemother. 2012; 67(5):1188-97, Bioorg Med Chem Lett, 2008, 18, 289-292)
| 1. DiaryloxyMethanoPhenanthrenes: A New Class of Antituberculosis Agents, G. Panda,Shagufta, Jitendra Kumar Mishra, Vinita Chaturvedi, Anil K. Srivastava, Manju, RanjanaSrivastava and Brahm S. Srivastava, 1178DEL2004 Filing date 24/06/04 | |
| 2. Thiophene containing Trisubstituted Methanes (TRSMs) as antitubercular agents, Gautam Panda, Maloy Kumar Parai, Priyanka Singh, Sudhir Sinha, Vinita Chaturvedi, Anil Gaikwad, PCT in process (685/DEL/2010) dt 20-2-2010 |
/////////
c1c(ccc(c1)OC)C(c2ccc(cc2)OCCN(C(C)C)C(C)C)c3sccc3
Filed under: Uncategorized Tagged: CDRI 830