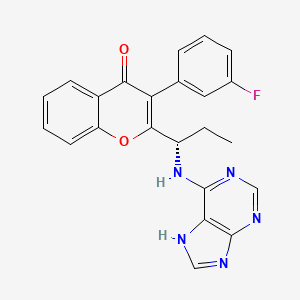
(S)-2-(l-(9H-purin-6-ylamino)propyl)-3-(3-fluorophenyl)-4H-chromen-4-one (Compound A1 is RP 6530).
RP 6530
RP 6530, RP6530, RP-6530
Tenalisib
RP6530-1401, NCI-2015-01804, 124584, NCT02567656
(S)-2-(l-(9H-purin-6-ylamino)propyl)-3-(3-fluorophenyl)-4H-chromen-4-one
3-(3-fluorophenyl)-2-[(1S)-1-(7H-purin-6-ylamino)propyl]chromen-4-one
MW415.4, C23H18FN5O2
CAS 1639417-53-0, 1693773-94-2
A PI3K inhibitor potentially for the treatment of hematologic malignancies.
An inhibitor of phosphoinositide-3 kinase (PI3K) δ/γ isoforms and anti-cellular proliferation agent for treatment of hematol. malignancies
Rhizen Pharmaceuticals is developing RP-6530, a PI3K delta and gamma dual inhibitor, for the potential oral treatment of cancer and inflammation In November 2013, a phase I trial in patients with hematologic malignancies was initiated in Italy ]. In September 2015, a phase I/Ib study was initiated in the US, in patients with relapsed and refractory T-cell lymphoma. At that time, the study was expected to complete in December 2016
PATENTS……..WO 11/055215 , WO 12/151525.
Inventors
| Inventors | Meyyappan Muthuppalaniappan, Srikant Viswanadha, Govindarajulu Babu, Swaroop Kumar V.S. Vakkalanka, |
| Incozen Therapeutics Pvt. Ltd., Rhizen… |
View original post 3,692 more words
Filed under: Uncategorized