
WO 2016042441, Mankind Research Centre, Silodosin, New patent


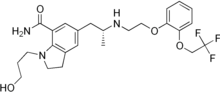
Mankind Research Centre
MANKIND RESEARCH CENTRE [IN/IN]; 191-E, Sector 4-II, IMT-Manesar, Haryana 122050 (IN)
A novel process for the preparation of considerably pure silodosin
GANGWAR, Kuldeep Singh; (IN).
KUMAR, Anil; (IN).
BHASHKAR, Bhuwan; (IN)
The present invention relates to a novel, improved, commercially viable and industrially advantageous process for the preparation of Silodosin of Formula (I), its pharmaceutically acceptable salts or solvates thereof. The invention relates to the preparation of considerably pure Silodosin with high yield.
Silodosin, l-(3-hydroxypropyl)-5-[(2R)-2-({2-[2-(2,2,2-trifluoroethoxy)phenoxy] ethyl} amino)propyl]-2,3-dihydro-lH-indole-7-carboxamide of Formula (I) is an indoline antidysuric which has a selectively inhibitory effect against urethra smooth muscle constriction, and decreases urethra internal pressure without great influence on blood pressure. Silodosin is available under trade names RAPAFLO® or UROREC®. Silodosin was first disclosed in EP 0600675 as a therapeutic agent for the treatment of dysuria associated with benign prostatic hyperplasia, where a process for producing the compound is also disclosed.

Formula (I)
Since, Silodosin is an optically active compound having a complex chemical structure; its synthesis is relatively complex and requires a sequence of multiple steps.
US patent no. 6,310,086, discloses a process for preparing Silodosin analogue compound from reaction of (R)-3-{5-(2-aminopropyl)-7-cyano-2,3-dihydro-lH-indol-l-yl} propylbenzoate with 2-(2-ethoxyphenoxy)ethyl methanesulfonate and finally isolated as a crude compound which is purified by column chromatography. The said process has a major disadvantage of using column chromatography which is not feasible at plant scale production.
PCT application no. WO 2012147019, discloses the preparation of Silodosin as shown in scheme- 1, wherein the Ν,Ν-dialkyl impurity of Formula (Ila) formed during condensation of 3-{7-cyano-5-[(2R)-2-aminopropyl]-2,3-dihydro-lH-indol-l-yl}propyl benzoate of Formula (III) with 2-(2-(2,2,2-trifluoroethoxy)phenoxy)ethyl methanesulfonate of Formula (IV); is removed through preparation of monotartarate salt to give compound of Formula (VI). The compound of Formula (VI) is base hydrolyzed followed by cyano hydrolysis to give crude Silodosin of Formula (VIII) which is then further purified by crystallization to get desired pure Silodosin.
Scheme- 1:

Major drawback of above said reaction process is that multiple isolations and crystallizations are required to get pure Silodosin.
Similarly, US 7,834,193 discloses monooxalate salt represented by Formula Via having 0.9% of dialkyl impurity represented by Formula Ila. The oxalate salt so obtained is subjected to alkaline hydrolysis followed by transformation of the nitrile to an amide.

Formula (Ila)
Similarly, PCT application no. WO 2012147107, discloses the method wherein Silodosin is prepared by condensation of 3-{7-cyano-5-[(2R)-2-aminopropyl]-2,3-dihydro-lH-indol-l-yl} propyl benzoate with 2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl methanesulfonate in solvent using base and phase transfer catalyst wherein, dialkyl impurity is formed up to 11%, followed by hydroxyl deprotection in protic solvent using base and phase transfer catalyst which is then subjected to purification to remove N,N-dialkyl impurity represented by Formula (lib) up to 0.6% through the preparation of acetate salt. This process suffers from a serious drawback i.e., accountable formation of dialkyl impurity and even after purification the impurity is reduced to only up to 0.6%. Secondly, the process requires multiple isolations and purifications ensuing into time engulfing workups and purifications and hence incurring solvent wastage. This makes process lengthy, uneconomical and tedious to be performed at plant scale.
Another PCT application no. WO 2012131710, discloses the preparation of Silodosin in which the chiral compound (3-(5-((R)-2-aminopropyl)-7-cyanoindolin-l-yl)propyl benzoate) is reacted with 2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl methane sulfonate in isopropyl alcohol using sodium carbonate as base. The reaction is completed in 40-50h and about 9-11% of dimer is formed during condensation. After completion of reaction, it is subjected to hydroxyl deprotection and the crude compound so obtained is purified to remove the Ν,Ν-dialkyl impurity of Formula (lib). The pure compound is then reacted with hydrogen peroxide in dimethyl sulfoxide to give Silodosin. The major drawback of this process is that the process is a multistep process wherein the condensation reaction is long-drawn-out resulting into countable amount of dimer formation during the process.
Thus, the prior art methods of preparing Silodosin require multiple and repeated purifications to synthesize DMF (Drug Master File) grade Silodosin. None of the prior art produces compound of Formula (VI) or (VII) with Ν,Ν-dialkyl impurity of Formula (Ila) or (lib) in an amount less than 0.6% to 0.5% even after purification. Therefore to prepare highly pure Silodosin, there is a need to explore new synthetic schemes that could be more economical and scalable. The present invention provides a novel, improved, commercially viable and industrially advantageous process for the synthesis of Silodosin and its pharmaceutically acceptable salts or solvates thereof. The present invention focus on preparation of highly pure Silodosin in appreciable yields with minimal use of solvents wherein the Silodosin is isolated with purity >99.5% having Ν,Ν-dialkyl impurity less than 0.03% and other individual impurities below 0.1%.

Ramesh Juneja (seated), founder of Mankind Pharma, with brother Rajeev, who is senior director (marketing & sales)
Mankind Pharma Chairman and Founder RC Juneja
In accordance to one embodiment of the present invention, the process of the preparation of Silodosin represented by Formula (I)

comprises the steps of:
a) condensing chiral compound represented by Formula (III)

Formula (III)
wherein, Bz represents to Benzoyl group with compound represented by Formula (IV)

Formula (IV)
wherein, Ms represents to Methanesulfonyl group in presence of base and phase transfer catalyst in an organic solvent to give intermediate represented by Formula (V)

Formula (V)
wherein, n is an integer of 1 and 2 and Bz is as defined above, wherein the compound having n=2 is formed in an amount of less than 5%;
b) optionally isolating compound of Formula (V),
c) without purification converting it to de-protected compound represented by Formula (IX) in an organic solvent;

Formula (IX)
wherein, n is as defined above;
d) optionally isolating compound of Formula (IX), and
e) without purification converting it to compound represented by Formula (X)

Formula (X)
wherein n is as defined above;
f) subjecting compound of Formula (X) to purification by converting to acid salt for removal of Ν,Ν-dialkyl impurity represented by Formula (lie);

Formula (He)
g) hydrolysis of the said acid salt to get Silodosin of Formula (I) with purity >99.5%.
Examples
The invention is explained in detail in the following examples which are given solely for the purpose of illustration only and therefore should not be construed to limit the scope of the invention.
Example 1
Preparation of Crude Silodosin:
Method A:
To the solution of lOg (0.0275 mol) of (3-(5-((R)-2-aminopropyl)-7-cyanoindolin-l-yl)propyl benzoate) in 100ml of toluene was added 14.3g (0.0826 mol) of dipotassium hydrogen phosphate and 8.20g (0.0261 mol) of 2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl methane sulfonate followed by addition of 2.0g (0.0055 mol) of tetrabutyl ammonium iodide and stirred the reaction mass at 85-90°C for 10-12h. Cooled the reaction mass, added de-mineralized water and separated the toluene layer followed by distillation to get crude viscous mass. Added 110ml of dimethyl sulfoxide and a solution of 1.51g (0.0415 mol) of sodium hydroxide dissolved in 8.52ml of water followed by addition of 6.42g (0.0567 mol) of 30% w/w of hydrogen peroxide. Stirred the reaction mass at 20-25°C till completion and added sodium sulfite solution. Extracted the compound in ethylacetate, washed the organic layer with brine solution and concentrated to get 10.2g of crude Silodosin.
Ν,Ν-dialkyl impurity is 3.2% as per HPLC.
Method B:
To the solution of lOg (0.0275 mol) of (3-(5-((R)-2-aminopropyl)-7-cyanoindolin-l-yl)propyl benzoate) in 100ml of toluene was added 14.3g (0.0826 mol) of dipotassium hydrogen phosphate and 8.20g (0.0261 mol) of 2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl methane sulfonate followed by addition of 2.0g (0.0055 mol) of tetrabutyl ammonium iodide and stirred the reaction mass at 85-90°C for 10-12h. Added solution of 4.4g of sodium hydroxide dissolved in 10ml of water and stirred the reaction at ambient temperature till completion. Quenched the reaction mass with water and separated the layers. Washed the toluene layer with brine and concentrated under reduced pressure to get crude mass. Dissolved the crude mass so obtained in 110ml of dimethyl sulfoxide and added a solution of 1.95g (0.0488 mol) of sodium hydroxide dissolved in 7.95ml of water followed by addition of 7.5g (0.066 mol) of 30% w/w of hydrogen peroxide. Stirred the reaction mass at room temperature followed by addition of 210ml of aqueous solution of sodium sulfite and extracted the compound in ethyl acetate. Washed the organic layer with brine and concentrated under reduced pressure to get 10. lg of crude Silodosin.
Ν,Ν-dialkyl impurity is 3.0% as per HPLC
Method C:
To the solution of lOg (0.0275 mol) of (3-(5-((R)-2-aminopropyl)-7-cyanoindolin-l-yl)propyl benzoate) in 100ml of dimethyl sulfoxide was added 14.3g (0.0826 mol) of dipotassium hydrogen phosphate and 8.20g (0.0261 mol) of 2-[2-(2,2,2-trifluoroethoxy)phenoxy] ethyl methane sulfonate followed by addition of 2.0g (0.0055 mol) of tetrabutyl ammonium iodide and stirred the reaction mass at 85-90°C for 2-3h. Added 100ml of water and 50ml of toluene and stirred the reaction mass at room temperature for half an hour. Separated the toluene layer and concentrated under reduced pressure. To the crude mass so obtained was added 110ml of dimethyl sulfoxide and a solution of 4.4g of sodium hydroxide dissolved in 10ml of water followed by addition of 7.5g (0.066 mol) of 30% w/w of hydrogen peroxide. Stirred the reaction mass at room temperature followed by addition of 210ml of aqueous solution of sodium sulfite and extracted the compound in ethyl acetate. Washed the organic layer with brine and concentrated under reduced pressure to get 9.8 g of crude Silodosin.
Ν,Ν-dialkyl impurity is 2.1% as per HPLC
Method D:
To the solution of 20g (0.055 mol) of (3-(5-((R)-2-aminopropyl)-7-cyanoindolin-l-yl)propyl benzoate) in 200ml of toluene was added 28.6g (0.165 mol) of dipotassium hydrogen phosphate and 16.4g (0.0522 mol) of 2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl methane sulfonate followed by addition of 4.0g (0.11 mol) of tetrabutyl ammonium iodide and stirred the reaction mass at 85-90°C for 10-12h. Added de-mineralized water and stirred at room temperature for half an hour. Separated the toluene layer to which was added a solution of 8.8g of sodium hydroxide dissolved in 20ml of water and stirred the reaction at ambient temperature till completion. Quenched the reaction mass with water and separated the layers. Washed the toluene layer with brine and concentrated under reduced pressure to get crude mass. Dissolved the crude mass so obtained in 200ml of dimethyl sulfoxide and added a solution of 3.9g (0.0976 mol) of sodium hydroxide dissolved in 16ml of water followed by addition of 15g (0.132 mol) of 30% w/w of hydrogen peroxide. Stirred the reaction mass at room temperature followed by addition of 400ml of aqueous solution of sodium sulfite and extracted the compound in ethyl acetate. Washed the organic layer with brine and concentrated under reduced pressure to get 21. Og of crude Silodosin.
Ν,Ν-dialkyl impurity is 2.8% as per HPLC
Method E:
To the solution of 2g (0.0055 mol) of (3-(5-((R)-2-aminopropyl)-7-cyanoindolin-l-yl)propyl benzoate) in 20ml of was dimethyl sulfoxide was added 2.87g (0.0165 mol) of dipotassium hydrogen phosphate and 1.64g (0.0052 mol) of 2-[2-(2,2,2-trifluoroethoxy)phenoxy] ethyl methane sulfonate followed by addition of 0.29g (0.0011 mol) of 16-crown ether and stirred the reaction mass at 85-90°C for 10-12h. Added a solution of 0.88g of sodium hydroxide dissolved in 2ml of water and stirred the reaction at ambient temperature till completion. Added de-mineralized water and toluene and stirred at room temperature for half an hour. Separated the toluene layer and concentrated under reduced pressure and to the solid mass so obtained were added 20ml of dimethyl sulfoxide and a solution of 0.38g (0.0231 mol) of sodium hydroxide dissolved in 1.6ml of water followed by addition of 1.5g (0.0132 mol) of 30% w/w of hydrogen peroxide. Stirred the reaction mass at room temperature followed by addition of aqueous solution of sodium sulfite and extracted the compound in ethyl acetate. Washed the organic layer with brine and concentrated under reduced pressure to get 2.1g of crude Silodosin.
Ν,Ν-dialkyl impurity is 2.2% as per HPLC
Method F:
To the solution of lOg (0.0275 mol) of (3-(5-((R)-2-aminopropyl)-7-cyanoindolin-l-yl)propyl benzoate) in 100ml of was acetonitrile was added 14.3g (0.0826 mol) of dipotassium hydrogen phosphate and 8.20g (0.0261 mol) of 2-[2-(2,2,2-trifluoroethoxy)phenoxy] ethyl methane sulfonate followed by addition of 2.0g (0.0055 mol) of tetra butyl ammonium iodide and stirred the reaction mass at 85-90°C for 10-12h. Added a solution of 4.4g of sodium hydroxide dissolved in 10ml of water and stirred the reaction at ambient temperature till completion. Added de-mineralized water and toluene and stirred at room temperature for half an hour. Separated the toluene layer and concentrated under reduced pressure and to the solid mass so obtained were added 110ml of dimethyl sulfoxide and a solution of 1.95g (0.0488 mol) of sodium hydroxide dissolved in 7.95ml of water followed by addition of 7.5g (0.066 mol) of 30% w/w of hydrogen peroxide. Stirred the reaction mass at room temperature followed by addition of 210ml of aqueous solution of sodium sulfite and extracted the compound in ethyl acetate. Washed the organic layer with brine and concentrated under reduced pressure to get 9.5g of crude Silodosin.
Ν,Ν-dialkyl impurity is 3.1% as per HPLC
Method G:
To the solution of lOg (0.0275 mol) of (3-(5-((R)-2-aminopropyl)-7-cyanoindolin-l-yl)propyl benzoate) in 100ml of was Dimethyl sulfoxide was added 14.3g (0.0826 mol) of dipotassium hydrogen phosphate and 8.20g (0.0261 mol) of 2-[2-(2,2,2-trifluoroethoxy)phenoxy] ethyl methane sulfonate followed by addition of 4.0g (0.0055 mol) of tetra butyl ammonium iodide and stirred the reaction mass at 85-90°C for 10-12h. Added a solution of 4.4g of sodium hydroxide dissolved in 10ml of water and stirred the reaction at ambient temperature till completion. Added de-mineralized water and toluene and stirred at room temperature for half an hour. Separated the toluene layer and concentrated under reduced pressure and to the solid mass so obtained were added 110ml of dimethyl sulfoxide and a solution of 1.95g (0.0488 mol) of sodium hydroxide dissolved in 7.95ml of water followed by addition of 7.5g (0.066 mol) of 30% w/w of hydrogen peroxide. Stirred the reaction mass at room temperature followed by addition of 210ml of aqueous solution of sodium sulfite and extracted the compound in ethyl acetate. Washed the organic layer with brine and concentrated under reduced pressure to get 10.4g of crude Silodosin.
Ν,Ν-dialkyl impurity is 1.83% as per HPLC
Example 2
Purification of Crude Silodosin:
To the lOg (0.0080 mol) of crude mass of Silodosin was added 110ml of isopropyl alcohol followed by addition of 1.75g of oxalic acid at ambient temperature. Stirred the solution 6-8h and filtered the precipitates. Added ethyl acetate and water in the ratio of 1: 1 to the above solid followed by addition of 5ml of liquor ammonia. Stirred the reaction mass at ambient temperature for 15 min and separated the layers. Concentrated the organic layer to ¼ of its volume and left undisturbed overnight. Filtered the precipitates and recrystallized with ethyl acetate followed by drying under reduced pressure to get 5.1g of pure Silodosin. The amount of impurities and the percent impurity of the Silodosin obtained was as follows:
Ν,Ν-dialkyl impurity: undetectable amount
Other impurities: 0.03 to 0.09%
Silodosin purity: 99.65% (HPLC)
////WO 2016042441, Mankind Research Centre, Silodosin, New patent
Filed under: PATENT, PATENTS Tagged: Mankind Research Centre, NEW PATENT, SILODOSIN, WO 2016042441
