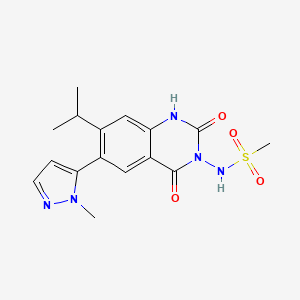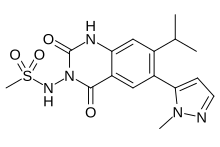
Selurampanel, BGG492,
cas 912574-69-7
Chemical Formula: C16H19N5O4S
Exact Mass: 377.1158
UNII-7WG1MR7DAR;
N-(7-isopropyl-6-(1-methyl-1H-pyrazol-5-yl)-2,4-dioxo-1,4-dihydroquinazolin-3(2H)-yl)methanesulfonamide
N-[7-Isopropyl-6-(1-methyl-1H-pyrazol-5-yl)-2,4-dioxo-1,2,3,4-tetrahydroquinazolin-3-yl]methanesulfonamide
PHASE 2 , FOR EPILEPSY, TITINUS
NOVARTIS INNOVATOR
Selurampanel (INN, code name BGG492) is a drug closely related to the quinoxalinedione series which acts as a competitive antagonist of the AMPA and kainate receptors and, as of 2015, is being investigated in clinical trials by Novartis for the treatment ofepilepsy.[1][2][3] It has also been studied in the acute treatment of migraine, and was found to produce some pain relief, but with a relatively high rate of side effects.[4]
PATENT
Example 44: N-[7-IsopropyI-6-(l-methyl-lH-pyrazol-4-yl)-2,4-dioxo-l,4-dihydro-2H-quinazoIin-3-yl]-methanesulfonamide
2-Amino-4-isopropyl-5-(2-methyl-2H-pyrazol-3-yl)-benzoic acid methyl ester

The 2-amino-5-iodo-4-isopropyl-benzoic acid methyl ester required for the coupling reaction described below was prepared according to the procedures described in WO 2004/033435 Al.
The l-methyl-5-tributylstannanyl-lH-pyrazole required for the coupling reaction was prepared according to the procedure described above.
2-Amino-5-iodo-4-isopropyl-benzoic acid methyl ester (300 mg, 0.94 mmol) and l-methyl-5-tributylstannanyl-lH-pyrazole (523 mg, 1.5 equiv) were weighed in air and added in a flame-dried flask. [Bistriphenylphosphine]dichloropalladium (67.3 mg, 0.1 equiv) was added and the flask was closed by a septum. Dioxane (1 mL) was added and the mixture was stirred for 18 h (TLC control) at 100 0C. The mixture was dissolved with EtOAc, filtered and evaporated to dryness. The crude product was purified by flash chromatography (hexanes to EtOAc / hexanes (4:6)) to yield 2-amino-4-isopropyl-5-(2-methyl-2H- pyrazol-3-yl)-benzoic acid methyl ester (169 mg, 66%) as a yellow solid. (ESI-MS: m/z 21 A [M+H]+, rt 5.20 min).
2-(4-Chloro-phenoxycarbonylamino)-4-isopropyl-5-(2-methyl-2H-pyrazol-3-yl)-benzoic acid methyl ester

4-Chlorophenyl-chloroformate (88 μL, 1.1 equiv) was added to a solution of 2-amino-4-isopropyl-5-(2~ methyl-2H-pyrazol-3-yl)-benzoic acid methyl ester (156 mg, 0.57 mmol) in dioxane (1.5 mL). The mixture was stirred for 2 h (TLC control) at 80 0C. The mixture was evaporated to dryness. The obtained yellow solid was used in the next step without further purification, (rt 6.77 min)
N-[7-Isopropyl-6-(2-methyl-2H-pyrazol-3 -yl)-2,4-dioxo- 1 ,4-dihydro-2H-quinazolin-3 -yl] -methanesulfonamide

CH3SO2NHNH2 (79.5 mg, 1.1 equiv) and J-Pr2NEt (225 μL, 2 equiv) were added to a solution of 2-(4-chloro-phenoxycarbonylamino)-4-isopropyl-5-(2-methyl-2H-pyrazol-3-yl)-benzoic acid methyl ester (281 mg, 0.65 mmol) in dioxane (8 mL). The mixture was stirred for 16 h (TLC control) at 80 0C. The mixture was evaporated to dryness. The crude product was purified by flash chromatography (MeOH / DCM (1:9)) to provide N-[7-isopropyl-6-(2-methyl-2H-pyrazol-3 ~yl)-2,4-dioxo- 1 ,4-dihydro-2H-quinazolin-3 -yl]-methanesulfonamide as a white solid (120 mg, 48%) (ESI-MS: m/z 378 [M+H]+, rt 4.20 min).
| Patent | Submitted | Granted |
|---|---|---|
| Substituted 1H-quinazoline-2,4-diones useful as AMPA receptor ligands [US7655666] | 2008-06-26 | 2010-02-02 |
| N-(2,4-dioxo-6-(tetrahydrofuran-2-yl)-7-(trifluoromethyl)-1,4-dihydro-2H-quinazolin-3-yl)methanesulfonamide [US8012988] | 2010-06-10 | 2011-09-06 |
| 2,4-DIOXO-1,4-DIHYDRO-2H-QUINAZOLIN-3-YL-SULFONAMIDE DERIVATIVES [US2013053381] | 2011-05-18 | 2013-02-28 |
| Use of 1H-quinazoline-2,4-diones [US2013090346] | 2012-09-05 | 2013-04-11 |
| Use of 1H-quinazoline-2,4-diones [US2013096145] | 2011-06-24 | 2013-04-18 |
| Use of 1H-quinazoline-2,4-diones [US2014163050] | 2014-02-12 | 2014-06-12 |
| FOMULATION COMPRISING 1 H-QUINAZOLINE-2, 4-DIONE AMPA RECEPTOR ANTAGONISTS, IN THE FORM OF IMMEDIATE RELEASE TABLETS AND PREPARATION THEREOF [US2012263791] | 2010-12-21 | 2012-10-18 |
| Use of 1H-Quinazoline-2,4-Diones [US2014018376] | 2010-10-20 | 2014-01-16 |
| 1-H-QUINAZOLINE-2, 4-DIONES FOR USE IN THE TREATMENT OF NEURONAL CEROID LIPOFUSCINOSIS [US2012122903] | 2010-07-23 | 2012-05-17 |
References
- Faught, Edward (2014). “BGG492 (selurampanel), an AMPA/kainate receptor antagonist drug for epilepsy”. Expert Opinion on Investigational Drugs 23 (1): 107–113.doi:10.1517/13543784.2014.848854. ISSN 1354-3784.
- Belcastro, Vincenzo; Verrotti, Alberto (2015). “Novel Molecular Targets for Drug-Treatment of Epilepsy”: 183–199.doi:10.1007/978-3-319-12283-0_10.
- Hanada, Takahisa (2014). “The AMPA receptor as a therapeutic target in epilepsy: preclinical and clinical evidence”. Journal of Receptor, Ligand and Channel Research: 39.doi:10.2147/JRLCR.S51475. ISSN 1178-699X.
- Gomez-Mancilla B, Brand R, Jürgens TP, et al. (February 2014). “Randomized, multicenter trial to assess the efficacy, safety and tolerability of a single dose of a novel AMPA receptor antagonist BGG492 for the treatment of acute migraine attacks”. Cephalalgia 34 (2): 103–13.doi:10.1177/0333102413499648. PMID 23963355.
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
N-[7-Isopropyl-6-(2-methylpyrazol-3-yl)-2,4-dioxo-1H-quinazolin-3-yl]methanesulfonamide
|
|
| Identifiers | |
| CAS Number | 912574-69-7 |
| ATC code | None |
| PubChem | CID 45381907 |
| ChemSpider | 32698379 |
| Chemical data | |
| Formula | C16H19N5O4S |
| Molar mass | 377.418 g/mol |
see……..http://apisynthesisint.blogspot.in/2016/02/selurampanel-bgg-492.html
////Selurampanel, BGG492, 912574-69-7
CC(C)c1cc2c(cc1c3ccnn3C)c(=O)n(c(=O)[nH]2)NS(=O)(=O)C
CS(=O)(NN1C(NC2=C(C=C(C3=CC=NN3C)C(C(C)C)=C2)C1=O)=O)=O
Filed under: Phase2 drugs Tagged: BGG 492, Epilepsy, oPHASE 2, Selurampanel, TITINUS