Image may be NSFW.
Clik here to view.
VAL-083
(1R,2S)-1-((R)-oxiran-2-yl)-2-((S)-oxiran-2-yl)ethane-1,2-diol
Galactitol, 1,2:5,6-dianhydro-
- 1,2:5,6-Dianhydrodulcitol
- 1,2:5,6-Dianhydrogalactitol
- 1,2:5,6-Diepoxydulcitol
Dianhydrodulcitol; Dianhydrogalactitol; VAL083; VAL 083, Dulcitol diepoxide, NSC 132313
CAS 23261-20-3
MF C6H10O4, MW 146.14
VAL-083 is a bi-functional alkylating agent; inhibit U251 and SF188 cell growth in monolayer better than TMZ and caused apoptosis
VAL-083 is a bi-functional alkylating agent, with potential antineoplastic activity. Upon administration, VAL-083 crosses the blood brain barrier (BBB) and appears to be selective for tumor cells. This agent alkylates and crosslinks DNA which ultimately leads to a reduction in cancer cell proliferation. In addition, VAL-083 does not show cross-resistance to other conventional chemotherapeutic agents and has a long half-life in the brain. Check for active clinical trials or closed clinical trials using this agent
Currently, VAL-083 is approved in China to treat chronic myelogenous leukemia and lung cancer, while the drug has also secured orphan drug designation in Europe and the US to treat malignant gliomas.
LAUNCHED CHINA FOR Cancer, lung
Del Mar Pharmaceuticals Inc……..Glioblastoma…………..PHASE2
DelMar and MD Anderson to accelerate development of anti-cancer drug VAL-083
DelMar Pharmaceuticals has collaborated with the University of Texas MD Anderson Cancer Center (MD Anderson) to speed up the clinical development of its VAL-083 anti-cancer drug.
Image may be NSFW.
Clik here to view.
VAL-083 is a BI-Functional alkylating agent; INHIBIT U251 and SF188 Cell Growth in monolayer Better than TMZ and Caused apoptosis. IC50 Value : 5 uM (INHIBIT U251, SF188, T98G Cell Growth in monolayer after 72h) [1]. in vitro :.. VAL-083 INHIBITED U251 and SF188 Cell Growth in monolayer and as neurospheres Better than TMZ and Caused apoptosis after 72 hr Formation Assay In the colony, VAL-083 (5 uM) SF188 Growth suppressed by about 95% are T98G cells classically TMZ-resistant and express MGMT, but VAL-083 inhibited their growth in monolayer after 72 hr in a dose-dependent manner (IC50, 5 uM). VAL-083 also inhibited the growth of CSCs (BT74, GBM4, and GBM8) . by 80-100% in neurosphere self-Renewal assays Conversely, there was minimal normal Effect on Human Neural stem cells [1]. in Vivo : Clinical Trial : Safety Study of VAL-083 in Patients With Recurrent Malignant glioma or Secondary Progressive Brain Tumor. Phase 1 / Phase 2
Image may be NSFW.
Clik here to view.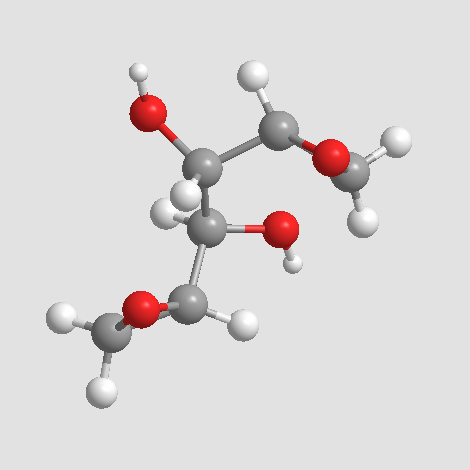
VAL-083 has demonstrated activity in cyclophosphamide, BCNU and phenylanine mustard resistant cell lines and no evidence of cross-resistance has been encountered in published clinical studies. Based on the presumed alkylating functionality of VAL-083, published literature suggests that DNA repair mechanisms associated with Temodar and nitrosourea resistance, such as 06-methylguanine methyltransferace (MGMT), may not confer resistance to VAL-083. VAL-083 readily crosses the blood brain barrier where it maintains a long half-life in comparison to the plasma. Published preclinical and clinical research demonstrates that VAL-083 is selective for brain tumor tissue. VAL-083 has been assessed in multiple studies as chemotherapy in the treatment of newly diagnosed and recurrent brain tumors. In published clinical studies, VAL-083 has previously been shown to have a statistically significant impact on median survival in high grade gliomas when combined with radiation vs. radiation alone. The main dose-limiting toxicity related to the administration of VAL-083 in previous clinical studies was myelosuppression
Image may be NSFW.
Clik here to view.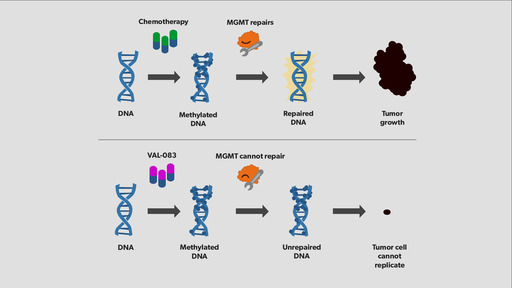
Glioblastoma is the most common form of primary brain cancer
DelMar Pharmaceuticals has collaborated with the University of Texas MD Anderson Cancer Center (MD Anderson) to speed up the clinical development of its VAL-083 anti-cancer drug.
VAL-083 is a small-molecule chemotherapeutic designed to treat glioblastoma multiforme (GBM), the most common and deadly cancer that starts within the brain.
Under the deal, MD Anderson will begin a new Phase II clinical trial with VAL-083 in patients with GBM at first recurrence / progression, prior to Avastin (bevacizumab) exposure.
During the trial, eligible patients will have recurrent GBM characterised by a high expression of MGMT, the DNA repair enzyme implicated in drug-resistance, and poor patient outcomes following current front-line chemotherapy.
The company noted that MGMT promoter methylation status will be used as a validated biomarker for enrollment and tumours must exhibit an unmethylated MGMT promoter for patients to be eligible for the trial.
DelMar chairman and CEO Jeffrey Bacha said: “The progress we continue to make with our research shows that VAL-083 may offer advantages over currently available chemotherapies in a number of tumour types.
“This collaboration will allow us to leverage world-class clinical and research expertise and a large patient population from MD Anderson as we extend and accelerate our clinical focus to include GBM patients, following first recurrence of their disease.
“We believe that VAL-083’s unique cytotoxic mechanism offers promise for GBM patients across the continuum of care as a potential superior alternative to currently available cytotoxic chemotherapies, especially for patients whose tumours exhibit a high-expression of MGMT.”
The deal will see DelMar work with the scientists and clinicians at MD Anderson to accelerate its research in order to transform the treatment of patients whose cancers fail or are unlikely to respond to existing treatments.
In more than 40 clinical trials, VAL-083 showed clinical activity against several cancers including lung, brain, cervical, ovarian tumours and leukemia both as a single-agent and in combination with other treatments.
PATENT
WO 2012024368
https://www.google.com/patents/WO2012024368A3?cl=en
Dianhydrogalactitol (DAG or dianhydrodulcitol) can be synthesized from dulcitol which can be produced from natural sources (such as Maytenus confertiflora) or commercial sources.The structure of DAG is given below as Formula (I).
One method for the preparation of dulcitol from Maytenus confertiflora is as follows: (1) The Maytenus confertiflora plant is soaked in diluted ethanol (50-80%) for about 24 hours, and the soaking solution is collected. (2) The soaking step is repeated, and all soaking solutions are combined. (3) The solvent is removed by heating under reduced pressure. (4) The concentrated solution is allowed to settle overnight and the clear supernatant is collected. (5) Chloroform is used to extract the supernatant. The chloroform is then removed under heat and reduced pressure. (6) The residue is then dissolved in hot methanol and cooled to allow crystallization. (7) The collected crystals of dulcitol are filtered and dried under reduced pressure. The purified material is dulcitol, contained in the original Maytenus confertiflora plant at a concentration of about 0.1% (1/1000).
DAG can be prepared by two general synthetic routes as described below:
Route 1 :
Dulcitol DAG
Route 2. Dulcitol
In Route 1 , “Ts” represents the tosyl group, or p-toluenesulfonyl group. PATENT
However, the intermediate of Route 1, 1,6-ditosy)dulcitol, was prepared with low yield (~36%), and the synthesis of 1,6-ditosyldulcitol was poorly reproducible. Therefore, the second route process was developed, involving two major steps: (1) preparation of dibromodulcitol from dulcitol; and (2) preparation of dianhydrodulcitol from dibromodulcitol.
Dibromodulcitol is prepared from dulcitol as follows: (1) With an aqueous HBr solution of approximately 45% HBr concentration, increase the HBr concentration to about 70% by reacting phosphorus with bromine in concentrated HBr in an autoclave. Cool the solution to 0° C. The reaction is:
2P+3Br2→2PBr3+H20→HBr†+H3P04. (2) Add the dulcitol to the concentrated HBr solution and reflux at 80° C to complete the reaction. (3) Cool the solution and pour the mixture onto ice water. Dibromodulcitol is purified through recrystallization.
The results for the preparation of dibromodulcitol (DBD) are shown in Table 1, below.
TABLE 1
For the preparation of DAG from DBD, DBD was poorly dissolved in methanol and ethanol at 40° C (different from what was described in United States PATENT
Patent No. 3,993,781 to Horvath nee Lengyel et al., incorporated herein by this reference). At refluxing, DBD was dissolved but TLC showed that new impurities formed that were difficult to remove from DBD.
The DBD was reacted with potassium carbonate to convert the DBD to dianhydrogalactitol.
The results are shown in Table 2, below.
TABLE 2
In the scale-up development, it was found the crude yield dropped significantly. It is unclear if DAG could be azeotropic with BuOH. It was confirmed that t-BuOH is essential to the reaction. Using MeOH as solvent would result in many impurities as shown spots on TLC. However, an improved purification method was developed by using a slurry with ethyl ether, which could provide DAG with good purity. This was developed after a number of failed attempts at recrystallization of DAG.
Image may be NSFW.
Clik here to view.
Bromination of dulcitol with HBr at 80°C gives dibromodulcitol , which upon epoxidation in the presence of K2CO3 in t-BuOH or NaOH in H2O or in the presence of ion exchange resin Varion AD (OH) (4) affords the target dianhydrogalactitol .
PATENT
Image may be NSFW.
Clik here to view.
Image may be NSFW.
Clik here to view.
Image may be NSFW.
Clik here to view.
SCHEME 5
Image may be NSFW.
Clik here to view. Image may be NSFW.
Image may be NSFW.
Clik here to view. Image may be NSFW.
Image may be NSFW.
Clik here to view.
PATENT
CN 103923039
http://www.google.com/patents/CN103923039A?cl=en
The resulting Dulcitol 9g and 18ml mass percent concentration of 65% hydrobromic acid at 78 ° C under reflux for 8 hours to give 1,6-dibromo dulcitol, and the product is poured into ice crystals washed anhydrous tert-butyl alcohol, and dried to give 1,6-dibromo dulcitol crystal, then 10.0gl, 6- dibromo dulcitol sample is dissolved in t-butanol, adding solid to liquid 2 % obtained through refining process 1,6_ dibromo dulcitol seed stirred and cooled to 0 ° C, allowed to stand for seven days to give 1,6_ dibromo dulcitol crystal, anhydrous t-butanol, dried to give 1,6-dibromo dulcitol. 5g of the resulting 1,6_ dibromo Euonymus dissolved in 50ml tert-butanol containing 5g of potassium carbonate, the elimination reaction, at 80 ° C under reflux time was 2 hours, the resulting product was dissolved in t-butanol, Join I% stock solution to the water quality of 1,2,4,5_ two Dulcitol including through a purification step to get less than 1% of 1,2,5,6_ two to water Dulcitol seeded stirring, cooling to 0 ° C, allowed to stand for I-day, two to go get 1,2,5,6_ water Dulcitol crystals washed anhydrous tert-butyl alcohol, and dried to give 1,2,5,6 two to crystalline water Dulcitol and lyophilized to give two to water Dulcitol lyophilized powder, containing I, 2,4,5- two to water Dulcitol less than 0.3%.
PATENT
PATENT
-
DAG can be prepared by two general synthetic routes as described below:
-
In Route 1, “Ts” represents the tosyl group, or p-toluenesulfonyl group.
-
However, the intermediate of Route 1, 1,6-ditosyldulcitol, was prepared with low yield (˜36%), and the synthesis of 1,6-ditosyldulcitol was poorly reproducible. Therefore, the second route process was developed, involving two major steps: (1) preparation of dibromodulcitol from dulcitol; and (2) preparation of dianhydrodulcitol from dibromodulcitol.
-
Dibromodulcitol is prepared from dulcitol as follows: (1) With an aqueous HBr solution of approximately 45% HBr concentration, increase the HBr concentration to about 70% by reacting phosphorus with bromine in concentrated HBr in an autoclave. Cool the solution to 0° C. The reaction is: 2P+3Br2→2PBr3+H2O→HBr↑+H3PO4. (2) Add the dulcitol to the concentrated HBr solution and reflux at 80° C. to complete the reaction. (3) Cool the solution and pour the mixture onto ice water. Dibromodulcitol is purified through recrystallization.
PATENT
US 20150329511
PAPER
Antibacterial and Anti-Quorum Sensing Molecular Composition Derived from Quercus cortex (Oak bark) Extract
Journal of the American Chemical Society, 1991 , vol. 113, 7 pg. 2786 – 2787
Clik here to view.

REFERENCES
Currently, VAL-083 is approved in China to treat chronic myelogenous leukemia and lung cancer, while the drug has also secured orphan drug designation in Europe and the US to treat malignant gliomas.
[1]. Fotovati A, Hu KJ, Wakimoto H, VAL-083, A NOVEL N7 ALKYLATING AGENT, SURPASSES TEMOZOLOMIDE ACTIVITY AND INHIBITS CANCER STEM CELLS, PROVIDING A NEW POTENTIAL TREATMENT OPTION FOR GLIOBLASTOMA MULTIFORME. Neuro-oncology, 2012, 14, AbsET-37, Suppl. 6
1: Szende B, Jeney A, Institoris L. The diverse modification of N-butyl-N-(4-hydroxybutyl) nitrosamine induced carcinogenesis in urinary bladder by dibromodulcitol and dianhydrodulcitol. Acta Morphol Hung. 1992;40(1-4):187-93. PubMed PMID: 1365762.
2: Anderlik P, Szeri I, Bános Z. Bacterial translocation in dianhydrodulcitol-treated mice. Acta Microbiol Hung. 1988;35(1):49-54. PubMed PMID: 3293340.
3: Huang ZG. [Clinical observation of 15 cases of chronic myelogenous leukemia treated with 1,2,5,6-dianhydrodulcitol]. Zhonghua Nei Ke Za Zhi. 1982 Jun;21(6):356-8. Chinese. PubMed PMID: 6957285.
4: Anderlik P, Szeri I, Bános Z, Wessely M, Radnai B. Higher resistance of germfree mice to dianhydrodulcitol, a lymphotropic cytostatic agent. Acta Microbiol Acad Sci Hung. 1982;29(1):33-40. PubMed PMID: 6211912.
5: Bános Z, Szeri I, Anderlik P. Effect of Bordetella pertussis vaccine on the course of lymphocytic choriomeningitis (LCM) virus infection in suckling mice pretreated with dianhydrodulcitol (DAD). Acta Microbiol Acad Sci Hung. 1979;26(2):121-5. PubMed PMID: 539467.
6: Bános Z, Szeri I, Anderlik P. Dianhydrodulcitol treatment of lymphocytic choriomeningitis virus infection in suckling mice. Acta Microbiol Acad Sci Hung. 1979;26(1):29-34. PubMed PMID: 484266.
7: Gerö-Ferencz E, Tóth K, Somfai-Relle S, Gál F. Effect of dianhydrodulcitol (DAD) on the primary immune response of normal and tumor bearing rats. Oncology. 1977;34(4):150-2. PubMed PMID: 335301.
8: Kopper L, Lapis K, Institóris L. Incorporation of 3H-dibromodulcitol and 3H-dianhydrodulcitol into ascites tumor cells. Autoradiographic study. Neoplasma. 1976;23(1):47-52. PubMed PMID: 1272473.
9: Bános S, Szeri I, Anderlik P. Combined phytohaemagglutinin and dianhydrodulcitol treatment of lymphocytic choriomeningitis virus infection in mice. Acta Microbiol Acad Sci Hung. 1975;22(3):237-40. PubMed PMID: 1155228.
Carbohydrate Research, 1982 , vol. 108, p. 173 – 180
Deryabin, Dmitry G.; Tolmacheva, Anna A.
Molecules, 2015 , vol. 20, 9 pg. 17093 – 17108
Gati; Somfai-Relle
Arzneimittel-Forschung/Drug Research, 1982 , vol. 32, 2 pg. 149 – 151
| WO2013128285A2 * | Feb 26, 2013 | Sep 6, 2013 | Del Mar Pharmaceuticals | Improved analytical methods for analyzing and determining impurities in dianhydrogalactitol |
| WO2013128285A3 * | Feb 26, 2013 | Dec 27, 2013 | Del Mar Pharmaceuticals | Improved analytical methods for analyzing and determining impurities in dianhydrogalactitol |
| US9029164 | Nov 18, 2013 | May 12, 2015 | Del Mar Pharmaceuticals | Analytical methods for analyzing and determining impurities in dianhydrogalactitol |
| US3470179 * | Jun 14, 1966 | Sep 30, 1969 | Sandoz Ag | 4-substituted-3,4-dihydroquinazolines |
| US20020032230 * | May 21, 2001 | Mar 14, 2002 | Dr. Reddy’s Laboratories Ltd. | Novel compounds having antiinflamatory activity: process for their preparation and pharmaceutical compositions containing them |
| US20020037328 * | May 31, 2001 | Mar 28, 2002 | Brown Dennis M. | Hexitol compositions and uses thereof |
| CN101045542A * | Apr 6, 2007 | Oct 3, 2007 | 中国科学院过程工程研究所 | Method for preparing water softening aluminium stone of sodium aluminate solution carbonation resolving |
| CN101654270A * | Sep 10, 2009 | Feb 24, 2010 | 沈阳工业大学 | Method for eliminating periodic thinning of granularity of seed product |
| CN101775413A * | Mar 23, 2010 | Jul 14, 2010 | 禹城绿健生物技术有限公司 | Technique for producing xylitol and dulcitol simultaneously |
| CN103270035A * | Aug 17, 2011 | Aug 28, 2013 | 德玛医药 | Method of synthesis of substituted hexitols such as dianhydrogalactitol |
/////////////////
C1C(O1)C(C(C2CO2)O)O
O[C@H]([C@H]1OC1)[C@@H](O)[C@H]2CO2
Image may be NSFW.
Clik here to view. DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
Filed under: 0rphan drug status, cancer, Phase2 drugs, Uncategorized Tagged: Dianhydrodulcitol, Dianhydrogalactitol, Orphan Drug Designation, phase 2, VAL 083 Image may be NSFW.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.







