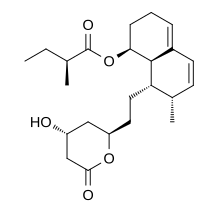
Mevastatin (compactin, ML-236B) is a hypolipidemic agent that belongs to the statins class.
It was isolated from the mold Penicillium citrinum by Akira Endo in the 1970s, and he identified it as a HMG-CoA reductase inhibitor,[1] i.e., a statin. Mevastatin might be considered the first statin drug;[2] clinical trials on mevastatin were performed in the late 1970s in Japan, but it was never marketed.[3] The first statin drug available to the general public was lovastatin.
In vitro, it has antiproliferative properties.[4]
A British group isolated the same compound from Penicillium brevicompactum, named it compactin, and published their results in 1976.[5] The British group mentions antifungal properties with no mention of HMG-CoA reductase inhibition.
High doses inhibit growth and proliferation of melanoma cells.[6]
| Systematic (IUPAC) name | |
|---|---|
| (1S,7R,8S,8aR)-8-{2-[(2R,4R)-4-Hydroxy-6-oxotetrahydro-2H-pyran-2-yl]ethyl}-7-methyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (2S)-2-methylbutanoate | |
| Clinical data | |
| Identifiers | |
73573-88-3  |
|
| None | |
| PubChem | CID: 64715 |
| IUPHAR/BPS | 3031 |
| DrugBank | DB06693  |
| ChemSpider | 58262  |
| UNII | 1UQM1K0W9X  |
| KEGG | C13963  |
| ChEBI | CHEBI:34848  |
| ChEMBL | CHEMBL54440  |
| Chemical data | |
| Formula | C23H34O5 |
| 390.513 g/mol | |
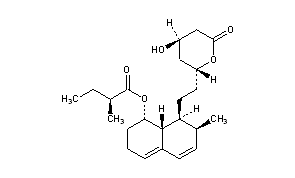 |
|
Title: Mevastatin
CAS Registry Number: 73573-88-3
CAS Name: (2S)-2-Methylbutanoic acid (1S,7S,8S,8aR)-1,2,3,7,8,8a-hexahydro-7-methyl-8-[2-[(2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl]ethyl]-1-naphthalenyl ester
Additional Names: 7-[1,2,6,7,8,8a-hexahydro-2-methyl-8-(methylbutyryloxy)naphthyl]-3-hydroxyheptan-5-olide; 2b-methyl-8a-(2-methyl-1-oxobutoxy)mevinic acid lactone; compactin; 6-demethylmevinolin
Manufacturers’ Codes: CS-500; ML-236 B
Molecular Formula: C23H34O5
Molecular Weight: 390.51
Percent Composition: C 70.74%, H 8.78%, O 20.49%
Literature References:
Fungal metabolite which is a potent inhibitor of HMG-CoA reductase, the rate controlling enzyme in cholesterol biosynthesis. Isoln from Penicillium citrinum: A. Endo et al., DE 2524355 corresp to US 3983140 (1975, 1976 to Sankyo).
Isoln from P. brevicompactum, crystal and molecular structure: A. G. Brown et al., J. Chem. Soc. Perkin Trans. 1 1976,1165.
Inhibition of HMG-CoA reductase activity: A. Endo et al., FEBS Lett. 72, 323 (1976); M. S. Brown et al., J. Biol. Chem. 253,1121 (1978).
Therapeutic effects in primary hypercholesterolemia: A. Yamamoto et al., Atherosclerosis 35, 259 (1980).
Total synthesis: N. Y. Wang et al., J. Am. Chem. Soc. 103, 6538 (1981); M. Hirama, M. Uei, ibid. 104, 4251 (1982); N. N. Girotra, N. L. Wendler, Tetrahedron Lett. 23, 5501 (1982); C.-T. Hsu et al., J. Am. Chem. Soc. 105, 593 (1983); P. A. Grieco et al., ibid. 1403; D. L. J. Clive et al., J. Am. Chem. Soc. 110, 6914 (1988). Review of syntheses: T. Rosen, C. H. Heathcock, Tetrahedron 42,4909-4951 (1986).
Review of mevastatin and related compounds: A. Endo, J. Med. Chem. 28, 401-405 (1985).
Properties: Crystals from aq ethanol, mp 152°. [a]D22 +283° (c = 0.48 in acetone). uv max: 230, 237, 246 nm (log e 4.28, 4.30, 4.11).
Melting point: mp 152°
Optical Rotation: [a]D22 +283° (c = 0.48 in acetone)
Absorption maximum: uv max: 230, 237, 246 nm (log e 4.28, 4.30, 4.11)
|
References
- Endo, Akira; Kuroda M.; Tsujita Y. (December 1976). “ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinium”. Journal of Antibiotics (Tokyo) 29 (12): 1346–8. doi:10.7164/antibiotics.29.1346. PMID 1010803.
- “The story of statins”.
- Endo, Akira (Oct 2004). “The origin of the statins”. Atheroscler Suppl. 5 (3): 125–30. doi:10.1016/j.atherosclerosissup.2004.08.033.PMID 15531285.
- Wachtershauser, A.; Akoglu, B; Stein, J (2001). “HMG-CoA reductase inhibitor mevastatin enhances the growth inhibitory effect of butyrate in the colorectal carcinoma cell line Caco-2″. Carcinogenesis 22 (7): 1061–7. doi:10.1093/carcin/22.7.1061. PMID 11408350.
- Brown, Allan G.; Smale, Terry C.; King, Trevor J.; Hasenkamp, Rainer; Thompson, Ronald H. (1976). “Crystal and molecular structure of compactin, a new antifungal metabolite from Penicillium brevicompactum.”. J. Chem. Soc., Perkin Trans. 1 (11): 1165–1170.doi:10.1039/P19760001165. PMID 945291.
- ^ Glynn, Sharon A; O’Sullivan, Dermot; Eustace, Alex J; Clynes, Martin; O’Donovan, Norma (2008). “The 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors, simvastatin, lovastatin and mevastatin inhibit proliferation and invasion of melanoma cells”. BMC Cancer8: 9. doi:10.1186/1471-2407-8-9. PMC 2253545. PMID 18199328.
The present invention
http://www.google.com/patents/US6204032
is related to a new method for producing ML-236B, a precursor of pravastatin sodium, in particular to a method for producing ML-236B lactone form(I), free acid form (II), and sodium salt(III) shown in the following formulae by using a new microorganism isolated from soil. ML-236B is obtained from the culture broth of this microorganism and it is used as a substrate of pravastatin sodium which is a potent cholesterol-lowering agent used in treatment for hypercholesterolemia.
2. Description of the Prior Art
It has been known that heart disease such as myocardial infarction, arteriosclerosis have been caused mainly by hyperlipidemia, especially hypercholesterolemia. It was reported by U.S. Pat. No. 3,983,140 and UK. Patent No. 1,453,425 that a cholesterol-lowering compound called ML-236B produced by a fungus Penicillium sp. had been discovered. ML-236B is produced by soil microorganisms or chemical conversion. It was reported that Penicillium brevicompactin, Penicilmyces sp., Trichoderma longibraiatum, Trichoderma pseudokoningi, Hyphomyces chrisopomus and Penicillium citrium produced ML-236B(David et al., “Biotechnology of filamentous fungi”, p241; JP Publication No. Pyung 4-349034).
Particularly, Sankyo Pharmaceutical Company, Japan, had developed Penicillium citrium SANK 18767 by mutation of a strain Penicillium citrium NRRL-8082 which was reported in 1971. By continuing strain development for 14 years, they had obtained Penicillium citrium Thom SANK 13380. ML-236B productivity had risen from 1.75 mg/l to 42.5 mg/l.
However, the method above described required so much time about 14 years to develop a strain with high ML-236B productivity. It also needed a little long cultivation time, 14 days, and showed relatively low ML-236B productivity.
The invention will be described in more detail in the drawings.
FIG. 1 is the IR spectrum of ML-236B obtained from this invention;

 and
and
FIG. 2 is the 13C-NMR spectrum of ML-236B obtained from this invention.

The physical properties such as appearance, melting point. molecular weight, elemental analysis, formular, UV spectrum, IR spectrum, solubility and specific rotation of ML-236B obtained from Example 2, 3 and Comparative Example are described in Table 1.
| TABLE 1 | ||
| COMPARATIVE | ||
| Article | EXAMPLE 2, 3 | EXAMPLE |
| Appearance | white crystal | white crystal |
| Melting point (° C.) | 150˜152 | 150˜152 |
| Molecular weight | calculated 390.2635 | experimental 390.2392 |
| experimental 390.2392 | ||
| Elemental | C 70.74, H 8.77, O 20.49 | C 70.74, O 20.49, H 8.77 |
| Analysis (%) | C 70.55 , H 8.69 | |
| calculated | C 70.85 , H 8.02 | |
| experimental | ||
| Formula | C23H34O5 | C23H34O5 |
| UV spectrum | 230, 237, 246 | 230, 237, 246 |
| (nm, MeOH) | ||
| IR spectrum | 3509, 2964, 2938, 2884, | 3509, 2964, 2938, 2884, |
| (cm−1, KBr ) | 1744, 1698, 1445, 1385, | 1744, 1699, 1445, 1385, |
| 1236, 1206, 1182, 1151, | 1236, 1206, 1182, 1150, | |
| 1077, 1056 | 1076, 1056 | |
| Solubility | methanol, chloroform, | methanol, chloroform, |
| soluble | ethanol, ethyl acetate | ethanol, ethyl acetate |
| insoluble | water | water |
| Specific rotation | +283n | +283n |
| [α]D | ||
13C NMR data of ML-236B are shown in Table 2 and FIG. 2.
| TABLE 2 | |||||
| The | δ c(ppm) | The | δ c(ppm) | ||
| number | EX- | COMPAR- | number | EX- | COMPAR- |
| of | AMPLE | ATIVE | of | AMPLE | ATIVE |
| carbon | 2,3 | EXAMPLE | carbon | 2,3 | EXAMPLE |
| C-1 | 171.50 | 170.67 | C-13 | 124.48 | 123.33 |
| C-2 | 39.31 | 38.44 | C-14 | 134.35 | 133.38 |
| C-3 | 63.18 | 62.12 | C-15 | 128.96 | 127.96 |
| C-4 | 36.88 | 35.84 | C-16 | 133.49 | 132.37 |
| C-5 | 77.22 | 76.26 | C-17 | 31.66 | 30.70 |
| C-6 | 33.75 | 32.82 | C-18 | 14.66 | 13.64 |
| C-7 | 24.83 | 23.78 | C-19 | — | — |
| C-8 | 37.66 | 36.67 | C-20 | 177.79 | 176.55 |
| C-9 | 38.31 | 37.40 | C-21 | 42.56 | 41.50 |
| C-10 | 68.45 | 67.51 | C-22 | 27.55 | 26.48 |
| C-11 | 27.06 | 26.30 | C-23 | 12.59 | 11.49 |
| C-12 | 21.74 | 20.74 | C-24 | 17.74 | 16.64 |
By using a new microorganism which was obtained from this invention, the productivity of pravastatin precursor was elevated highly and the pravastatin precursor could be prepared in a simple way in short time.
Therefore, the present invention could be used effectively in production of pravastatin precursor.
DR ANTHONY MELVIN CRASTO

MY BLOGS ON MED CHEM

FLAGS AND HITS
…………..today on this blog
Filed under: Uncategorized Tagged: compactin, Mevastatin, ML-236B



