
C20H22N8O6S2
534.57
209467-52-7
(6R,7R)-7-[(2Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-2-(N-hydroxyimino)acetamido]-8-oxo-3-{[(3E,3’R)-2-oxo-[1,3′-bipyrrolidin]-3-ylidene]methyl}-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
- BAL-9141
- BAL-9141-000
- BAL-9141000
- BAL9141-000
- RO 63-9141
- RO-63-9141
- RO-639141
ceftobiprole medocaril sodium
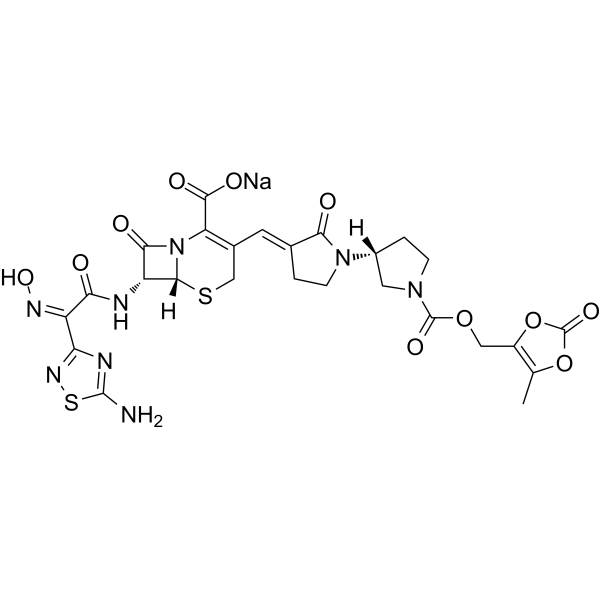

| Molecular Weight | 712.64 |
|---|---|
| Formula | C26H25N8NaO11S2 |
| CAS No. | 252188-71-9 |
| Appearance | Solid |
| Color | Off-white to light yellow |
| SMILES | O=C(C(N(C1=O)[C@@](SC2)([H])[C@@H]1NC(/C(C3=NSC(N)=N3)=N\O)=O)=C2/C=C(CCN4[C@@](CC5)([H])CN5C(OCC(OC6=O)=C(O6)C)=O)/C4=O)O[Na] |
fda approved 4/3/2024, To treat certain bloodstream infections, bacterial skin and associated tissue infections, and community-acquired bacterial pneumonia
Press Release zevtera
Ceftobiprole, sold under the brand name Zevtera among others, is a fifth-generation[5] cephalosporin antibacterial used for the treatment of hospital-acquired pneumonia (excluding ventilator-associated pneumonia) and community-acquired pneumonia. It is marketed by Basilea Pharmaceutica under the brand names Zevtera and Mabelio.[6][7][8][9][10][11] Like other cephalosporins, ceftobiprole exerts its antibacterial activity by binding to important penicillin-binding proteins and inhibiting their transpeptidase activity which is essential for the synthesis of bacterial cell walls. Ceftobiprole has high affinity for penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus strains and retains its activity against strains that express divergent mecA gene homologues (mecC or mecALGA251). Ceftobiprole also binds to penicillin-binding protein 2b in Streptococcus pneumoniae (penicillin-intermediate), to penicillin-binding protein 2x in Streptococcus pneumoniae (penicillin-resistant), and to penicillin-binding protein 5 in Enterococcus faecalis.[12]
Medical uses
In the US, ceftobiprole is indicated for the treatment of adults with Staphylococcus aureus bloodstream infections (bacteremia) including those with right-sided infective endocarditis;[4] adults with acute bacterial skin and skin structure infections;[4] and people with community-acquired bacterial pneumonia.[4]
Microbiology
Ceftobiprole has shown in vitro antimicrobial activity against a broad range of Gram-positive and Gram-negative pathogens. Among the Gram-positive pathogens, ceftobiprole has demonstrated good in vitro activity against methicillin-resistant Staphylococcus aureus, methicillin-susceptible Staphylococcus aureus and coagulase-negative staphylococci, as well as against strains of methicillin-resistant Staphylococcus aureus with reduced susceptibility to linezolid, daptomycin or vancomycin.[13] Ceftobiprole has also displayed potent activity against Streptococcus pneumoniae (including penicillin-sensitive, penicillin-resistant and ceftriaxone-resistant strains) and Enterococcus faecalis, but not against Enterococcus faecium. For Gram-negative pathogens, ceftobiprole has shown good in vitro activity against Haemophilus influenzae (including both ampicillin-susceptible and ampicillin-non-susceptible isolates), Pseudomonas aeruginosa and strains of Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis that do not produce extended-spectrum β-lactamases (ESBL). Like all other cephalosporins, ceftobiprole was inactive against strains that produce extended-spectrum β-lactamases.[14]
The efficacy of ceftobiprole has been demonstrated in two large randomized, double-blind, phase 3 clinical trials in patients with hospital-acquired and community-acquired pneumonia. Ceftobiprole was non-inferior to ceftazidime plus linezolid in the treatment of hospital-acquired pneumonia (excluding ventilator-acquired pneumonia) and non-inferior to ceftriaxone with or without linezolid in the treatment of community-acquired pneumonia.[15][16]
Pharmacology


Ceftobiprole is the active moiety of the prodrug ceftobiprole medocaril and is available for intravenous treatment only. It is mainly excreted via the kidney.[17]
Society and culture
Legal status

Ceftobiprole has been approved for the treatment of adults with hospital acquired pneumonia (excluding ventilator-acquired pneumonia) and community-acquired pneumonia in twelve European countries, Canada, and Switzerland.[18]
In February 2010, the Committee for Medicinal Products for Human Use of the European Medicines Agency adopted a negative opinion, recommending the refusal of the marketing authorization for the medicinal product Zeftera, intended for treatment of complicated skin and soft-tissue infections in adults. The company that applied for authorization is Janssen-Cilag International N.V. The applicant requested a re-examination of the opinion. After considering the grounds for this request, the CHMP re-examined the opinion, and confirmed the refusal of the marketing authorization in June 2010.[19]
syn

https://www.sciencedirect.com/topics/neuroscience/ceftobiprole
syn
WO2010136423
Processes for producing ceftobiprole medocaril are known per se. What the processes known from the prior art have in common is that, starting from 7-aminocephalosporanic acid, a large number of intermediates have to be produced, isolated and purified in order to obtain ceftobiprole medocaril of the general formula (1) in sufficient purity.
The compound of the general formula (1) is known per se and is described, for example, in WO 99/65920. It can be used for the treatment and prophylaxis of bacterial infectious diseases, especially infectious diseases caused by methicillin-resistant Staphylococcus Aureus strains.
WO 99/65920 describes, as the last step in the production process of ceftobiprole Medocaril, a reaction in which the Medocaril prodrug unit is introduced into a compound of the general formula (2).

The compound of the general formula (2) is also known per se and has been described, for example, in EP 0 849 269 A1. The compound of the general formula (2) is prepared according to EP 0 849 269 A1 starting from (2R,6R,7R)-te rt. B u toxyc abonylamin o-3-formyl-8-oxo-5-thia-1 -azabicyclo[4.2.0]oct-3-ene-2-carboxylic acid benzhydryl ester by Wittig reaction with (1 ‘-allyloxycarbonyl-2- oxo-[1,3’]bipyrrolidinyl-3-yl)-triphenylphosphonium bromide. The resulting Δ2 reaction product is reisomerized to the desired Δ3 isomer by sulfoxidation and subsequent reduction and then deprotected from the benzhydryl ester with trifluoroacetic acid. The acylation in position 7 occurs by reaction with (Z)-(5-amino-[1,2,4]-thiadiazol-3-yl)-trityloxyiminothioacetic acid S-benzothiazol-2-yl ester. The compound of the general formula (2) is then obtained by removing the protective groups.
In EP 1 067 131 A1 the formation of the ylide in toluene or a mixture of toluene and dichloromethane is tert by adding alkali. Butylate in tetrahydrofuran, which allows the base to be added as a solution. The reaction of the ylide with the corresponding aldehyde is described at a reaction temperature of -70 0 C.
EP 0 841 339 A1 relates to cephalosporin derivatives and processes for their production. WO 95/29182 also discloses intermediates for the production of cephalosporins.
WO 01/90111 describes a further production of ceftobiprole Medocaril in several stages starting from desacetyl-7-aminocephalosporanic acid by acylation with (Z)-(5-amino-[1,2,4]-thiadiazol-3-yl)- trityloxyiminothioacetic acid S-benzothiazol-2-yl ester in N,N-dimethylformamide, followed by in situ esterification with diphenyldiazomethane in dichloromethane to give the corresponding benzohydryl ester, which is precipitated and isolated by adding hexane. In the next step, this product is oxidized to the corresponding aldehyde using TEMPO/NaOCI in dichloromethane/water or with Braunstein in tetrahydrofuran/dichloromethane. The next reaction step involves the Wittig reaction to the 3-vinyl-substituted derivative, in which the reaction takes place in dichloromethane/toluene/tetrahydrofuran at -78°C. The crude product is stirred with ethanol and made from dichloromethane/tert. Butyl methyl ether recrystallized or purified chromatographically. According to the method disclosed in WO 01/901 11, the Wittig reaction is carried out at low temperatures of -80 to -70 0 C in a complex solvent mixture of dichloromethane, toluene and tetrahydrofuran. This leads to significant disadvantages when carrying out the reaction on a production scale, since regeneration of the process solvents is difficult.
EXAMPLES
1. Example: (6R,7R)-7-Amino-3[E-(R)-1′-(5-tert-butyloxycarbonyl)-2-oxo-[I.S’lbipyrrolidinyl-S-ylidenemethyll-β- oxo-S-thia-i -aza-bicyclo^^.Oloct^-ene-2-carboxylic acid
5 , 1 4 g of 7-amino-3-formyl-ceph-3-em-4-carboxy I at was dissolved in 2 7 , 8 m bis(trimethylsilyl)acetamide and 50 ml propylene oxide. 16.8 g of (1 R/S,3’R)-(1′-tert-butyloxycarbonyl-2-oxo-[1,3′]bipyrrolidinyl-3-yl)-triphenylphosphonium bromide (EP1067131, WO02/14332) slowly added in portions. Stirring was continued at 1 ° C until the starting material had reacted and then the crystalline precipitate was added
Nitrogen atmosphere filtered off and washed with 50 ml cyclohexane/bis(tirmethylsilyl)acetamide 99.5/0.5. After drying under vacuum, the desired product was obtained in silylated form.
The material was dissolved in 100ml dichloromethane and at 0 0 C with 50ml 3%
NaHCC> 3 solution added. The phases were separated, the organic phase was washed with 30 ml of water and the combined water phases were adjusted to pH 3.5 with 3% H 3 PO 4 after activated carbon treatment. The crystalline precipitate was filtered, washed with water and dried under vacuum.
Auswaage: 6,09g
1H-nmr(DMSO-d6) δ 1.39(s,9H), 2.00(m, 2H), 2.8-3.2(m, 2H), 3.2-3.5(m,6H), 3.84(ABq, 2H, J= 18.2Hz), 4.57(m,1H), 4.82(d,1H, J=5.1Hz), 5.01 (d,1H, J=5.1Hz), 7.21 (m,1H)
13 C-nmr(DMSO-d 6 ) d 24.63, 26.11 , 28.09, 28.89, 41.54, 44.94, 45.31 , 47.98, 48.34, 51.27, 52.00, 58.98, 63.76, 79.95, 121.95 , 126.19, 126.28, 129.90, 134.21, 154.97 , 164.36, 169.05, 169.13
MS- ESI negative mode: 927.2(2M-H, 100%, 463.1(M-H, 25%)
H2O content: 2.2 %
IR (golden gate, cm“1): 2978, 1793, 1682, 1551, 1397, 1363, 1330
2. Beispiel: (6R,7R)-7-Trimethylsilylamino-3[E-(R)-1′-(5-tert.butyloxycarbonyl)-2- oxo-[1 , 3′]bipyrrolidinyl -3 -ylidenemethyll-δ-oxo-δ-thia-i-aza-bicyclo^^.0]oct- 2-ene-2-carbonsäure trimethylsilylester
10.28 g of 7-amino-3-formyl-ceph-3-em-4-carboxylate were dissolved in 55.6 ml of bis(trimethylsilyl)acetamide and 100 ml of propylene oxide. 33.6 g of (1R/S, 3’R)-(1′-tert. Butyloxycarbonyl-2-oxo-[1,3′]bipyrrolidinyl-3-yl)-triphenylphosphonium bromide (EP1067131, WO02.) were then added at 0 0 C /14332) slowly added in portions over 22 hours. The mixture was stirred at 1° C. until the starting material had reacted and then the reaction mixture was cooled to -20 ° C. The crystalline precipitate was filtered off under a nitrogen atmosphere and washed in portions with 180 ml of cyclohexane/bis(trimethylsilyl)acetamide 99.5/0.5. After drying under vacuum, the bissilylated
Product received.
Weight: 16.2g
1H-nmr(CDCI3) δ 0.04,0.10,0.12(3s, 9H), 0,34(s, 9H), 1,43 (s, 9H), 1.74 (br s, 1H),
1.9-2.2 (m, 2H), 2.8-3.0 (m,2H), 3.2-3.7 (m,8H), 4.7-4.95 (m, 3H), 7.43 (m, 1H)
3. Beispiel: Dicyclohexylammonium (6R,7R)-7-Amino-3[E-(R)-1′-(5-tert. butyloxycarbonyl)-2-oxo-[1 ,3′]bipyrrolidinyl-3-ylidenemethyl]-8-oxo-5-thia-1 – aza-bicyclo[4.2.0]oct-2-ene-2-carboxylat
1.0g (6R,7R)-7-Trimethylsilylamino-3[E-(R)-1′-(5-tert.butyloxycarbonyl)-2-oxo-[I.Slbipyrrolidinyl-S-ylidenemethyO-δ-oxo-δ -thia-i-aza-bicyclo^^.Oloct^-ene^- carboxylic acid trimethylsilyl esters were dissolved in 10ml dichloromethane and a solution of 300mg dicyclohexylamine in 1ml EtOH and 10ml ethyl acetate was added. The precipitate was filtered off, washed with ethyl acetate and dried in vacuo.
Auswaage: 0,9g 1H-nmr(D2O/DMSO-d6) δ 0.9-1 .3(m, 10H), 1 .30(s,9H), 1 .4-2.18m,12H), 2.7- 3.5(01,1 OH), 3.64(ABq, J= 17.2Hz, 2H), 4.5 (m,1 H) * , 4.58 (d,1 H, J=5.1 Hz); 4.88 (d,1 H, J=5.1 Hz), 7.07 (s,1 H)
* partly overlaid by D20 signal
MS- ESI negative mode: 927.2(2M-H, 100%), 463.1 (M-H, 25%)
IR (golden gate, cm “1): 2932, 2856, 1754, 1692, 1671 , 1630, 1569, 1394, 1329
4. Specifically: (6R, 7R )-7-[(Z)-2-(5-Amino-[1 ,2,4]thiadiazol-3-yl)-2-hydroxyimino- acetylamino]-8-oxo- 3-[(E)-(R)-2-oxo-[1 , 3′]bipyrrolidinyl-3-ylidenemethyl]-5-thia-1-aza-bicyclo[4.2.0]oct-2-jen-2 carbons Trifluoroacetate
4.1 Variant A:
3.0g (6R,7R)-7-Amino-3[E-(R)-1′-(5-tert-butyloxycarbonyl)-2-oxo-[1,3′]bipyrrolidinyl-3-ylidenemethyl]-8 -oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid in silylated form was dissolved in 150 ml of dichloromethane at 0°. 600 μl of DMF/water 5/1 and 1.8 ml of bis(trimethylsilyl) acetamide and 2.29 g of 2-trityloxyimino-2-(5-amino-1,2,4-thiadiazol-3-yl) acetic acid chloride were then added Hydrochoride (J. Antibiotics 37:557 – 571, 1984) was added in portions.
After 3 hours at 0°, the mixture was poured into 30 ml MeOH/120 ml water and the methylene chloride phase was separated off. The organic phase was concentrated to 66g and 25ml of trifluoroacetic acid was added. After 10 minutes, 1.5 ml triethylsilane and 10 ml water were added and the mixture was cooled to -15 ° C. The organic phase was separated off and again with 6 ml
Washed trifluoroacetic acid/water 1/1. The combined aqueous phases were diluted to 150 ml with water and filtered through an adsorber resin column with XAD-1600. After washing out the column with water, elution was carried out with water/acetonitrile 85/15. The product-containing fractions were concentrated in vacuo and allowed to stand at 0° for post-crystallization. The crystalline
Product was filtered off, washed with water and dried under vacuum.
Auswaage: 2,66g
4.2 Variant B:
7.4g (6R,7R)-7-Amino-3[E-(R)-1′-(5-tert-butyloxycarbonyl)-2-oxo-[1,3′]bipyrrolidinyl-3-ylidenemethyl]-8 -oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid was dissolved at 0° in 781 ml of dichloromethane with the addition of 6.7 ml of triethylamine. 8.65 g of 2-trityloxyimino-2-(5-amino-1,2,4-thiadiazol-3-yl)-acetic acid chloride hydrochloride were then added in portions. After the starting material had reacted, the mixture was poured into 500 ml of water and the methylene chloride phase was separated off. The organic phase was dried over Na 2 SC> 4 and concentrated in vacuo.
The residue was dissolved in 148 ml of dichloromethane and 4.5 ml of triethylsilane and 74 ml of trifluoroacetic acid were added at room temperature. After 30 minutes, 222 ml of dichloromethane and 222 ml of water were added and the mixture was cooled to -20 0 C. The organic phase was separated off and washed again with a mixture of 37 ml of trifluoroacetic acid and 148 ml of water. The combined aqueous phases were diluted with water to 364 ml, filtered through an adsorber resin and eluted with acetonitrile/water 15/85.
The filtrate was concentrated to 35g on a Rotavapor, filtered and washed with water.
After drying in a vacuum, 4.5 g of the sample was obtained.
1H-nmr(DMSO-d6) δ 1.9-2.2(m,2H), 2.8-3.5(m, 8H), 3.85(Abq, 2H; J=18.3Hz), 4.63(m,1 H), 5.16(d, 2H, J=4.9Hz), 5.85(dd, 1 H, J1 = 4.9Hz, J2=8.4Hz), 7.23(s, 1 H),
8.06(s, 2H), 9.08 (br. s, 2H), 9.49(d, 2H, J=8.4Hz), 1 1.95 (s, 1 H)
5. Being typical: (6R.7R )-7-[(Z)-2-(5-Amino-[1 ,2,4]thiadiazol-3-yl)-2-hydroxyimino- acetylamino]- 8-oxo- . 3-[(E)-(R)-2-oxo-[1 ,3′]bipyrrolidinyl-3-ylidenemethyl]-5- thia-1 -aza-bicyclo[4.2.0]oct-2-jan-2 carbons
6.0g (6R,7R)-7-Amino-3[E-(R)-1′-(5-tert-butyloxycarbonyl)-2-oxo-[1,3′]bipyrrolidinyl-3-ylidenemethyl]-8 -oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid in silylated form was dissolved in 300 ml of dichloromethane at 0°. 1200 μl of DMF/water 5/1 and 8.1 ml of bis(tirmethylsilyl)acetamide were then added as well as 5.3g of 2-trityloxyimino-2-(5-amino-1,2,4-thiadiazol-3-yl)acetic acid chloride Hydrochoride (J. Antibiotics 37:557 – 571, 1984) added in portions. The mixture was then poured into 60 ml MeOH/240 ml water and the methylene chloride phase was separated off. The organic phase was concentrated to 48g and 1.5 ml of triethylsilane was added. After adding 50ml
Trifluoroacetic acid was stirred at room temperature for 60 min, 20 ml of water was added and the mixture was cooled to -15°C. The organic phase was separated off and washed again with 20 ml trifluoroacetic acid/water 1/1. The combined aqueous phases were diluted to 500 ml with water and treated with 2.0 g of activated carbon. After filtration, the solution was concentrated in vacuo.
The residue was diluted to 50 ml with water and adjusted to pH 6.9 with saturated NaHCO 3 solution. The mixture was stirred at 0 0 C for 2 hours, filtered and the precipitate washed with water.
Auswaage: 4,5g
1H-nmr(DMSO-d6/CF3COOD) δ 1.9-2.3(m,2H), 2.8-3.5(m,8H), 3.85(ABq, 2H, 18.7Hz), 4.61 (m,1 H), 5.16(d, 1 H,J=4.8Hz), 5.86(dd, 1 H,J1 =4.8Hz, J2=8.4Hz),
7.24(s,1 H), 8.05(br s, 2H), 8.93(s, 2H), 9.50(d,1 H,J=8.4Hz), 11.96(s, 1 H)
MS- ESI negative mode: 533.2(M-H, 10%)
6. Beispiel: Ceftobiprol Medocaril Na-SaIz
0, 5 3 g ( 6 R , 7 R )-7-[(Z)-2-(5-amino-[1,2,4]thiadiazol-3-yl)-2-hydroxyimino-acetylamino]-8- oxo-3-[(E)-(R)-2-oxo-[1,3′]bipyrrolidinyl-3-ylidenemethyl]-5-thia-1 – aza-bicyclo[4.2.0]oct-2-ene- 2 carboxylic acid were dissolved in 5 ml of dimethyl sulfoxide and 0.27 g of carbonic acid (5-methyl-2-oxo-[1,3]dioxol-4-ylmethyl)-4-nitrophenyl ester were added and stirred at room temperature. A solution of sodium ethyl hexanoate in 30 ml of acetone was added for precipitation. The precipitate was filtered and washed with acetone.
Auswaage: 0,6g
1H-nmr(DMSO-d6) δ 1.9-2.05(m, 2H), 2.10(s,3H), 2.7-3.1 (m,2H), 3.1-3.6(m,6H), 3.64(q, 2H; J=17.1 Hz), 4.56(m,1 H), 4.87(s,2H), 4.98(d,1 H,J=4.9Hz), 5.65(dd,1 H,J1 =4.9Hz, J2=8.4Hz), 7.34(s,1 H), 8.02(s,2H), 9.36(d,1 H,J=8.4Hz)
MS- ESI negative mode: 689.0(M-H, 100%)
| Clinical data | |
|---|---|
| Trade names | Zevtera, Mabelio |
| Other names | RO0639141-000,[1] BAL9141,[2] ceftobiprole medocaril |
| AHFS/Drugs.com | International Drug Names |
| License data | US DailyMed: Ceftobiprole |
| Routes of administration | Intravenous |
| Drug class | Cephalosporin antibacterial |
| ATC code | J01DI01 (WHO) |
| Legal status | |
| Legal status | AU: S4 (Prescription only)[3]UK: POM (Prescription only)US: ℞-only[4]In general: ℞ (Prescription only) |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 209467-52-7 252188-71-9 |
| PubChem CID | 6918430 |
| DrugBank | DB04918 |
| ChemSpider | 23350302 |
| UNII | 5T97333YZK |
| KEGG | D08885 |
| ChEMBL | ChEMBL520642 |
| CompTox Dashboard (EPA) | DTXSID40870229 |
| ECHA InfoCard | 100.129.666 |
| Chemical and physical data | |
| Formula | C20H22N8O6S2 |
| Molar mass | 534.57 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| hideSMILESC1CNC[C@@H]1N2CC/C(=C\C3=C(N4[C@@H]([C@@H](C4=O)NC(=O)/C(=N\O)/c5nc(sn5)N)SC3)C(=O)O)/C2=O | |
| hideInChIInChI=1S/C20H22N8O6S2/c21-20-24-14(26-36-20)11(25-34)15(29)23-12-17(31)28-13(19(32)33)9(7-35-18(12)28)5-8-2-4-27(16(8)30)10-1-3-22-6-10/h5,10,12,18,22,34H,1-4,6-7H2,(H,23,29)(H,32,33)(H2,21,24,26)/b8-5+,25-11-/t10-,12-,18-/m1/s1 Key:VOAZJEPQLGBXGO-SDAWRPRTSA-N | |
| (what is this?) (verify) | |
References
- ^ Hebeisen P, Heinze-Krauss I, Angehrn P, Hohl P, Page MG, Then RL (March 2001). “In vitro and in vivo properties of Ro 63-9141, a novel broad-spectrum cephalosporin with activity against methicillin-resistant staphylococci”. Antimicrobial Agents and Chemotherapy. 45 (3): 825–836. doi:10.1128/AAC.45.3.825-836.2001. PMC 90381. PMID 11181368.
- ^ Jones RN, Deshpande LM, Mutnick AH, Biedenbach DJ (December 2002). “In vitro evaluation of BAL9141, a novel parenteral cephalosporin active against oxacillin-resistant staphylococci”. The Journal of Antimicrobial Chemotherapy. 50 (6): 915–932. doi:10.1093/jac/dkf249. PMID 12461013.
- ^ “Prescription medicines: registration of new chemical entities in Australia, 2015”. Therapeutic Goods Administration (TGA). 21 June 2022. Archived from the original on 10 April 2023. Retrieved 10 April 2023.
- ^ Jump up to:a b c d https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/218275s000lbl.pdf
- ^ Scheeren TW (1 January 2015). “Ceftobiprole medocaril in the treatment of hospital-acquired pneumonia”. Future Microbiology. 10 (12): 1913–1928. doi:10.2217/fmb.15.115. PMID 26573022.
- ^ “Basilea announces distribution agreement with Cardiome to commercialize antibiotic Zevtera/Mabelio (ceftobiprole) in Europe and Israel”. Basilea (Press release). 12 September 2017. Retrieved 7 April 2024.
- ^ “Basilea to launch Zevtera/Mabelio (ceftobiprole medocaril) in Europe through a commercial services provider” (Press release). Basilea Pharmaceutica. Archived from the original on 31 March 2019. Retrieved 20 September 2016.
- ^ “Basilea announces launch of antibiotic Zevtera (ceftobiprole medocaril) in Germany”. Basilea (Press release). 5 December 2014. Retrieved 7 April 2024.
- ^ “Swissmedic approves Basilea’s antibiotic Zevtera (ceftobiprole medocaril) for the treatment of pneumonia”. Basilea (Press release). 22 December 2014. Retrieved 7 April 2024.
- ^ “Basilea signs exclusive distribution agreement for Zevtera (ceftobiprole medocaril) in the Middle East and North Africa with Hikma Pharmaceuticals LLC”. Basilea (Press release). 15 October 2015. Retrieved 7 April 2024.
- ^ “Basilea announces that Health Canada approved Zevtera for the treatment of bacterial lung infections”. Basilea (Press release). 12 October 2015. Retrieved 7 April 2024.
- ^ Syed YY (September 2014). “Ceftobiprole medocaril: a review of its use in patients with hospital- or community-acquired pneumonia”. Drugs. 74 (13): 1523–1542. doi:10.1007/s40265-014-0273-x. PMID 25117196. S2CID 2925496.
- ^ Zhanel GG, Lam A, Schweizer F, Thomson K, Walkty A, Rubinstein E, et al. (2008). “Ceftobiprole: a review of a broad-spectrum and anti-MRSA cephalosporin”. American Journal of Clinical Dermatology. 9 (4): 245–254. doi:10.2165/00128071-200809040-00004. PMID 18572975. S2CID 24357533.
- ^ Farrell DJ, Flamm RK, Sader HS, Jones RN (July 2014). “Ceftobiprole activity against over 60,000 clinical bacterial pathogens isolated in Europe, Turkey, and Israel from 2005 to 2010”. Antimicrobial Agents and Chemotherapy. 58 (7): 3882–3888. doi:10.1128/AAC.02465-14. PMC 4068590. PMID 24777091.
- ^ Farrell DJ, Flamm RK, Sader HS, Jones RN (April 2014). “Activity of ceftobiprole against methicillin-resistant Staphylococcus aureus strains with reduced susceptibility to daptomycin, linezolid or vancomycin, and strains with defined SCCmec types”. International Journal of Antimicrobial Agents. 43 (4): 323–327. doi:10.1016/j.ijantimicag.2013.11.005. PMID 24411474.
- ^ Nicholson SC, Welte T, File TM, Strauss RS, Michiels B, Kaul P, et al. (March 2012). “A randomised, double-blind trial comparing ceftobiprole medocaril with ceftriaxone with or without linezolid for the treatment of patients with community-acquired pneumonia requiring hospitalisation”. International Journal of Antimicrobial Agents. 39 (3): 240–246. doi:10.1016/j.ijantimicag.2011.11.005. PMID 22230331.
- ^ Awad SS, Rodriguez AH, Chuang YC, Marjanek Z, Pareigis AJ, Reis G, et al. (July 2014). “A phase 3 randomized double-blind comparison of ceftobiprole medocaril versus ceftazidime plus linezolid for the treatment of hospital-acquired pneumonia”. Clinical Infectious Diseases. 59 (1): 51–61. doi:10.1093/cid/ciu219. PMC 4305133. PMID 24723282.
- ^ “Zevtera 500 mg powder for concentrate for solution for infusion – Summary of Product Characteristics (SmPC)”. (emc). 5 April 2023. Retrieved 1 June 2023.
- ^ “Zeftera (previously Zevtera) EPAR”. European Medicines Agency (EMA). 18 February 2010. Retrieved 6 April 2024. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
External links
- Clinical trial number NCT03138733 for “Ceftobiprole in the Treatment of Patients With Staphylococcus Aureus Bacteremia” at ClinicalTrials.gov
- Clinical trial number NCT03137173 for “Ceftobiprole in the Treatment of Patients With Acute Bacterial Skin and Skin Structure Infections” at ClinicalTrials.gov
- Clinical trial number NCT00326287 for “Ceftobiprole in the Treatment of Patients With Community-Acquired Pneumonia” at ClinicalTrials.gov
- Clinical trial number NCT03439124 for “Ceftobiprole in the Treatment of Pediatric Patients With Pneumonia” at ClinicalTrials.gov
////////Ceftobiprole, BAL-9141, BAL-9141-000, BAL-9141000, BAL9141-000, RO 63-9141, RO-63-9141, RO-639141, fda 2024, zevtera, approvals 2024, Ceftobiprole medocaril sodium salt
[H][C@@]1(NC(=O)C(=N/O)\C2=NSC(N)=N2)C(=O)N2C(C(O)=O)=C(CS[C@]12[H])\C=C1/CCN(C1=O)[C@]1([H])CCNC1