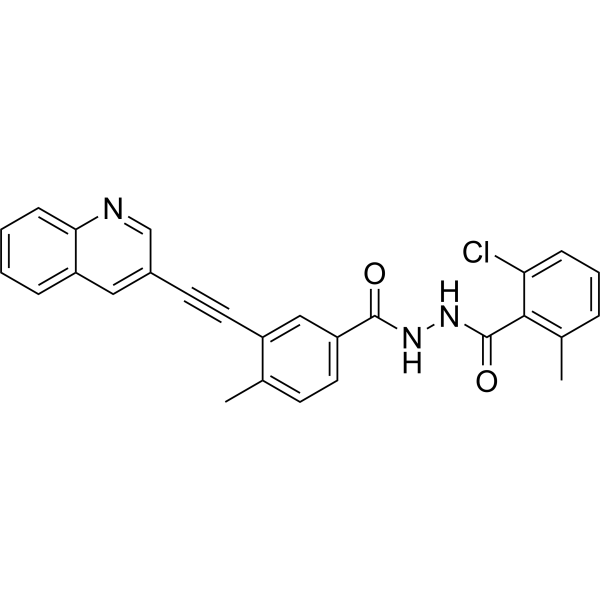
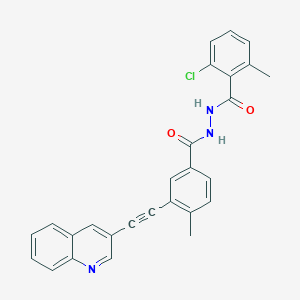
VODOBATINIB
1388803-90-4
| Molecular Weight | 453.92 |
|---|---|
| Appearance | Solid |
| Formula | C27H20ClN3O2 |
- SCO-088
- K0706
- K-0706
2-chloro-6-methyl-N‘-[4-methyl-3-(2-quinolin-3-ylethynyl)benzoyl]benzohydrazide
Vodobatinib (K0706) is a potent, third generation and orally active Bcr-Abl1 tyrosine kinase inhibitor with an IC50 of 7 nM. Vodobatinib exhibits activity against most BCR-ABL1 point mutants, and has no activity against BCR-ABL1T315I. Vodobatinib can be used for chronic myeloid leukemia (CML) research. Vodobatinib is a click chemistry reagent, itcontains an Alkyne group and can undergo copper-catalyzed azide-alkyne cycloaddition (CuAAc) with molecules containing Azide groups.
| Vodobatinib (K0706) is a potent, third generation and orally active Bcr-Abl1 tyrosine kinase inhibitor with an IC50 of 7 nM. Vodobatinib exhibits activity against most BCR-ABL1 point mutants, and has no activity against BCR-ABL1T315I. Vodobatinib can be used for chronic myeloid leukemia (CML) research[1][2]. Vodobatinib is a click chemistry reagent, itcontains an Alkyne group and can undergo copper-catalyzed azide-alkyne cycloaddition (CuAAc) with molecules containing Azide groups. |
Brain penetrant kinase inhibitors: Learning from kinase neuroscience discovery
Publication Name: Bioorganic & Medicinal Chemistry Letters
Publication Date: 2018-06-15
PMID: 29752185
DOI: 10.1016/j.bmcl.2018.05.007
PATENT
WO2012098416
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2012098416
EXAMPLES
Reƒerence Example 1
Methyl 3-ethynyl-4-methylbenzoate


A mixture of methyl 3-iodo-4-methylbenzoate (2.0g, 7mmol), trimethylsilylacetylene (1.2ml, 8mmol), Pd(PPh3)4 (0.42g, 0.3mmol), CuI (0.137g, 0.7mmol) and diisopropylethylamine (2.5ml, 11.4mmol) in THF (20ml) was heated at 50°C for 12hrs under nitrogen atmosphere. The reaction mixture was cooled to ambient temperature and filtered through a Celite® bed. The clear filtrate was concentrated and the residue purified by flash chromatography on silica gel (elution with 2% ethyl acetate in n-hexane) to provide methyl 4-methyl-3-[(trimethylsilyl)ethynyl]benzoate.
To the solution of methyl 4-methyl-3-[(trimethylsilyl)ethynyl]benzoate (2.3g) in THF (40ml) was added tetrabutylammonium fluoride (1.0M in THF, 3.2ml, 1 1mmol) at ambient
temperature and stirred for 15 minutes, concentrated and the residue purified by flash chromatography on silica gel (elution with 2% ethyl acetate in n-hexane) to provide methyl 3 – ethynyl- 4-methylbenzo at e .
1H NMR (500 MHz in DMSO-d6), δ 2.50 (s, 3H), 3.90 (s, 3H), 4.57 (s, 1H), 7.51 (d, J = 8.0 Hz, 1H), 7.91 (d, J = 8.0 Hz, 1H), 7.99 (s, 1H).
Similarly were prepared the following ester compounds from their corresponding iodo esters:
Methyl 3-ethynyl-4-fluorobenzoate
Methyl 3-ethynyl-4-methoxybenzoate
Reƒerence Example 2
4-Methyl-3-[(quinolin-3-yl)ethynyl]benzoic acid


A mixture of methyl 3-ethynyl-4-methylbenzoate (0.341 g, 2mmol), 3-iodoquinoline (0.5g, 2mmol), Pd(PPh3)4 (0.1 1g, 0.01mmol), CuI (0.179g, 0.1mmol) and diisopropylethylamine (0.5ml, 3mmol) in DMF (15ml) was stirred at ambient temperature for 12hrs under an atmosphere of nitrogen. The reaction mixture was concentrated and the crude product was purified by flash chromatography on silica gel (elution with 10% ethyl acetate in n-hexane) to provide methyl 4-methyl-3-[(quinolin-3-yl)ethynyl]benzoate.
Sodium hydroxide (0.15g, 3.71mmol) was added to a solution of the above methyl ester in methanol (20ml) and water (3ml) and stirred at 50°C for 3hrs and then concentrated in vacuo. Water (10ml) was added to the residue, adjusted pH to 4.0-4.5 with citric acid. The solid obtained was filtered, washed successively with water and diethyl ether and dried at ambient temperature to obtain 4-methyl-3-[(quinolin-3-yl)ethynyl]benzoic acid. 1H NMR (500 MHz in DMSO-d6), δ 2.66 (s, 3H), 7.56 (d, J = 8.0 Hz, 1H), 7.75 (t, J; = 15.1 Hz, J2 = 8.2 Hz, 1H), 7.89 (t, J} = 13.7 Hz, J2 = 8.5 Hz, 1H), 7.95 (d, J = 8.0 Hz, 1H), 8.09 (d, J = 8.2 Hz, 1H), 8.12 (d, J = 8.1 Hz, 1H), 8.17 (s, 1H), 8.75 (s, 1H), 9.1 1 (s, 1H), 12.84 (s, 1H).
Reƒerence Example 3
4-Methyl-3-[2-(3-quinolyl)ethynyl]benzohydrazide


A mixture of 4-methyl-3-[(quinolin-3-yl)ethynyl]benzoic acid (0.15g, 0.5mmol), N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (0.15g, 0.7mmol) and 1-hydroxybenzotriazole (0.1g, 0.7mmol) in N,N-dimethylformamide (15ml) was stirred at room temperature for 1hr. Hydrazine hydrate (1.52ml, 0.5mmol) was then added and the mixture stirred for another 3hrs. Concentration and trituration of the residue with water produced a solid which was filtered, washed successively with water and diethyl ether, and finally dried in vacuo to get the hydrazide as a pale yellow solid.
1H NMR (400 MHz in DMSO-d6), δ 2.63 (s, 3H), 4.79 (s, 2H), 7.51 (d, J = 8.0 Hz, 1H), 7.75 (t, J1 = 14.7 Hz, J2 = 7.6 Hz, 1H), 7.85-7.96 (m, 2H), 8.09-8.13 (m, 3H), 8.73 (s, 1H), 9.09 (s, 1H), 9.91 (s, 1H).
Reƒerence Example 4
N’-(3-iodo-4-methylbenzoyl)-2,4,6-trichlorobenzohydrazide


N’-(3-iodo-4-methylbenzoyl)-2,4,6-trichlorobenzohydrazide was prepared by the reaction of 3-iodo-4-methylbenzoic acid with 2,4,6-trichlorobenzohydrazide. The coupling was performed in a manner similar to that described in Reference Example 3.
Example 1.1
2,4,6-Trichloro-N’-[4-methyl-3-[2-(3-quinolyl)ethynyl]benzoyl]benzohydrazide
Method A:


A mixture of 4-methyl-3-[(quinolin-3-yl)ethynyl]benzoic acid (0.15g, 0.5mmol), N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (0.15g, 0.7mmol) and 1-hydroxybenzotriazole (0.1g, 0.7mmol) in N,N-dimethylformamide (15ml) was stirred at ambient temperature for 1hr. 2,4,6-Trichlorobenzohydrazide (0.125g, 0.5mmol) was added and the mixture stirred for 12hrs at ambient temperature. Concentration and trituration of the residue with water produced a solid which was filtered, washed with water and the crude product was purified by flash chromatography on silica gel (elution with 10% methanol in dichloromethane) to get 2,4,6-trichloro-N-[4-methyl-3-[2-(3-quinolyl)ethynyl]benzoyl] benzohydrazide as a white solid.
Method B:
2,4,6-Trichloro-N’-[4-methyl-3-[2-(3-quinolyl)ethynyl]benzoyl] benzohydrazide was also prepared by the reaction of 4-methyl-3-[(quinolin-3-yl)ethynyl]benzoic acid with 2,4,6-trichlorobenzohydrazide in diethyl cyanophosphonate. The condensation reaction was performed in a manner similar to that described in Method A.
Method C:


2,4,6-Trichloro-N-[4-methyl-3-[2-(3-quinolyl)ethynyl]benzoyl]benzohydrazide was also prepared by the reaction of 4-methyl-3-[(quinolin-3-yl)ethynyl]benzohydrazide with 2,4,6- trichlorobenzoyl chloride. The condensation reaction was performed in a manner similar to that described in Method A.
The compounds 1.2 to 1.14, 1.21 to 1.34, 1.36 to 1.40, and 1.43 to 1.59 were prepared in a manner similar to Example I.1, by following either of the methods A, B or C, using the appropriate substrates.
PATENT
WO2023214314 VODOBATINIB FOR REDUCING PROGRESSION OF PARKINSON’S DISEASE (wipo.int)
Vodobatinib (N’-(2-chloro-6-methylbenzoyl)-4-methyl-3-[2-(3-quinolyl) ethynyl]-benzohydrazide), a c-Abl inhibitor is represented by Formula I (referred hereinafter interchangeably as vodobatinib or compound of Formula
International Publication Nos. WO 2017/208267A1, WO 2020/250133 Al and WO 2022/024072A1, which are hereby incorporated by reference, disclose methods of use of the compound of Formula I for the treatment of Parkinson’s disease, synucleinopathies and Alzheimer’s disease (AD) respectively.
There is a continuing need for effective and safe methods for the treatment of, and delaying the progression of, neurodegenerative diseases, including in the early-stage of the diseases.
- N’-(2-chloro-6-methylbenzoyl)-4-methyl-3-[2-(3-quinolyl) ethynyl]-benzohydrazide for treatment of alzheimer’s diseasePublication Number: WO-2022024072-A1Priority Date: 2020-07-31
- Compounds for the treatment of covid-19Publication Number: EP-3875078-A1Priority Date: 2020-03-06
- Treatment for synucleinopathiesPublication Number: US-2022257582-A1Priority Date: 2019-06-11
- Novel amorphous dispersion of 4-Methyl-3-quinolin-3-ylethynyl-benzoic acid N’-(2-chloro-6-methyl-benzoyl) hydrazidePublication Number: AU-2018235446-A1Priority Date: 2017-03-15
- Novel amorphous dispersion of 4-methyl-3-quinolin-3-ylethynyl-benzoic acid n’-(2-chloro-6-methyl-benzoyl) hydrazidePublication Number: EP-3596050-A1Priority Date: 2017-03-15
- Novel amorphous dispersion of 4-methyl-3-quinolin-3-ylethynyl-benzoic acid n’-(2-chloro-6-methyl-benzoyl) hydrazidePublication Number: US-2020085751-A1Priority Date: 2017-03-15
- Novel amorphous dispersion of 4-methyl-3-quinolin-3-ylethynyl-benzoic acid n’-(2-chloro-6-methyl-benzoyl) hydrazidePublication Number: WO-2018167802-A1Priority Date: 2017-03-15
- NOVEL AMORPHOUS DISPERSION OF 4-METHYL-3-QUINOLIN-3-YETHYNYL-BENZOIC ACID HYDRAZIDE N- (2-CHLORO-6-METHYL-BENZOYL)Publication Number: WO-2018167802-A9Priority Date: 2017-03-15
- Novel amorphous dispersion of 4-methyl-3-quinolin-3-ylethynyl-benzoic acid n’-(2-chloro-6-methyl-benzoyl) hydrazidePublication Number: US-2021267906-A1Priority Date: 2017-03-15
- Novel amorphous dispersion of 4-Methyl-3-quinolin-3-ylethynyl-benzoic acid N’-(2-chloro-6-methyl-benzoyl) hydrazidePublication Number: AU-2018235446-B2Priority Date: 2017-03-15Grant Date: 2022-04-07
- Amorphous dispersion of 4-methyl-3-quinolin-3-ylethynyl-benzoic acid n’-(2-chloro-6-methyl-benzoyl) hydrazidePublication Number: US-11351123-B2Priority Date: 2017-03-15Grant Date: 2022-06-07
- Treatment of parkinson’s diseasePublication Number: US-2019275017-A1Priority Date: 2016-06-02
- Treatment of Parkinson’s diseasePublication Number: US-10849887-B2Priority Date: 2016-06-02Grant Date: 2020-12-01
- Treatment of Parkinson’s diseasePublication Number: IL-263188-APriority Date: 2016-06-02
- Treatment of Parkinson’s diseasePublication Number: CN-109475539-BPriority Date: 2016-06-02Grant Date: 2021-12-28
- Treatment of Parkinson’s diseasePublication Number: JP-6974357-B2Priority Date: 2016-06-02Grant Date: 2021-12-01
- Treatment for parkinson’s diseasePublication Number: US-2022273632-A1Priority Date: 2016-06-02
- Diarylacetylene hydrazide containing tyrosine kinase inhibitorsPublication Number: AU-2012208388-A1Priority Date: 2011-01-21
- Diarylacetylene hydrazide containing tyrosine kinase inhibitorsPublication Number: AU-2012208388-A2Priority Date: 2011-01-21
- Diarylacetylene hydrazide containing tyrosine kinase inhibitorsPublication Number: EP-2665709-B1Priority Date: 2011-01-21Grant Date: 2016-12-07
- Tyrosine kinase inhibitors containing diarylacetylene hydrazidePublication Number: ES-2608829-T3Priority Date: 2011-01-21Grant Date: 2017-04-17
- Diarylacetylene hydrazide containing tyrosine kinase inhibitorsPublication Number: US-2013296557-A1Priority Date: 2011-01-21
- Diarylacetylene hydrazide containing tyrosine kinase inhibitorsPublication Number: US-9024021-B2Priority Date: 2011-01-21Grant Date: 2015-05-05
//////////////


AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
///////////
Ref
- [1]. Orlando Antelope, et al. BCR-ABL1 tyrosine kinase inhibitor K0706 exhibits preclinical activity in Philadelphia chromosome-positive leukemia. Exp Hematol. 2019 Sep;77:36-40.e2. [Content Brief][2]. Phase 1 Trial of Vodobatinib, a Novel Oral BCR-ABL1 Tyrosine Kinase Inhibitor (TKI): Activity in CML Chronic Phase Patients Failing TKI Therapies Including Ponatinib. Session: 632: Chronic Myeloid Leukemia: Therapy: CML: New and Beyond.
///////VODOBATINIB, SCO-088, K0706, K-0706
CC1=C(C(=CC=C1)Cl)C(=O)NNC(=O)C2=CC(=C(C=C2)C)C#CC3=CC4=CC=CC=C4N=C3