
Adagrasib
| Formula | C32H35ClFN7O2 |
|---|---|
| cas | 2326521-71-3 |
| Mol weight | 604.1174 |
| Antineoplastic | |
| Disease | Non-small cell lung cancer |
|---|
| 2022/12/12 |
FDA APPROVED, KRAZATI (Mirati Therapeutics)
- MRTX-849
- MRTX849
- KRAS G12C inhibitor MRTX849
Adagrasib, sold under the brand name Krazati, is an anticancer medication used to treat non-small cell lung cancer.[1][2] Adagrasib is an inhibitor of the RAS GTPase family.[1] It is taken by mouth.[1] It is being developed by Mirati Therapeutics.[1][3]
The most common adverse reactions include diarrhea, nausea, fatigue, vomiting, musculoskeletal pain, hepatotoxicity, renal impairment, dyspnea, edema, decreased appetite, cough, pneumonia, dizziness, constipation, abdominal pain, and QTc interval prolongation.[2] The most common laboratory abnormalities include decreased lymphocytes, increased aspartate aminotransferase, decreased sodium, decreased hemoglobin, increased creatinine, decreased albumin, increased alanine aminotransferase, increased lipase, decreased platelets, decreased magnesium, and decreased potassium.[2]
It was approved for medical use in the United States in December 2022.[1][3]
Synthesis Reference
Fell, Jay B et al. “Identification of the Clinical Development Candidate MRTX849, a Covalent KRASG12C Inhibitor for the Treatment of Cancer.” Journal of medicinal chemistry vol. 63,13 (2020): 6679-6693. doi:10.1021/acs.jmedchem.9b02052
Journal of Medicinal Chemistry (2020), 63(13), 6679-6693
PATENT
WO2020101736 https://patents.google.com/patent/WO2020101736A1/en
EXAMPLE 7

2-[(2S)-4-[7-(8-chloro-1-naphthyl)-2-[[(2S)-1-methylpyrrolidin-2-yl]methoxy]-6,8-dihydro-5H- pyrido[3,4-d]pyrimidin-4-yl]-1-(2-fluoroprop-2-enoyl)piperazin-2-yl]acetonitrile
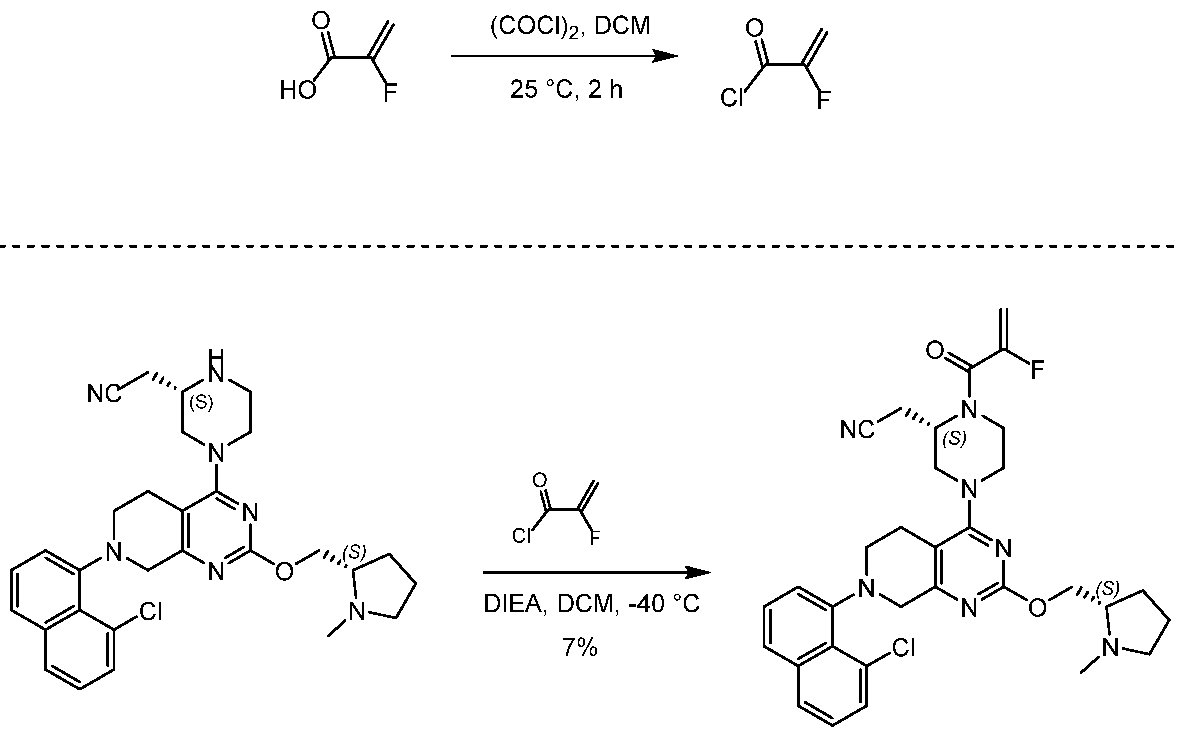
[0432] 2-fluoroprop-2-enoyl chloride. To a solution of 2-fluoroprop-2-enoic acid (400 mg, 4.44 mmol, 1 eq) in DCM (4 mL) was added (COCl)2 (846 mg, 6.66 mmol, 583 µL, 1.5 eq) and DMF (32.5 mg, 444 umol, 34.2 µL, 0.1 eq). The mixture was stirred at 25 °C for 2 hrs. The reaction mixture was concentrated under reduced pressure to remove a part of solvent and give a residue in DCM. Compound 2-fluoroprop-2-enoyl chloride (400 mg, crude) was obtained as a yellow liquid and used into the next step without further purification. [0433] Step A: 2-[(2S)-4-[7-(8-chloro-1-naphthyl)-2-[[(2S)-1- methylpyrrolidin-2-yl]methoxy]- 6,8-dihydro-5H-pyrido[3,4-d]pyrimidin-4-yl]-1-(2-fluoroprop-2-enoyl)piperazin-2- yl]acetonitrile. To a solution of 2-[(2S)-4-[7-(8-chloro-1-naphthyl)-2-[[(2S)- 1-methylpyrrolidin- 2-yl]methoxy]-6,8-dihydro-5H-pyrido[3,4-d]pyrimidin-4-yl]piperazin-2-yl]acetonitrile (300 mg, 528 umol, 1 eq, HCl) in DCM (5 mL) was added DIEA (1.73 g, 13.4 mmol, 2.33 mL, 25.4 eq) and 2-fluoroprop-2-enoyl chloride (286 mg, 2.64 mmol, 5 eq) in DCM (5 mL). The mixture was stirred at 0 °C for 1 hour. The reaction mixture was concentrated under reduced pressure to give a residue. The residue was purified by column chromatography (Al2O3, Dichloromethane/Methanol = 10/1 to 10/1). The residue was purified by prep-HPLC (column: Gemini 150 * 25 5u; mobile phase: [water (0.05% ammonia hydroxide v / v) – ACN]; B%: 55% – 85%, 12min). The residue was purified by prep-HPLC (column: Phenomenex Synergi C18 150 * 30mm * 4um; mobile phase: [water (0.225% FA) – ACN]; B%: 20% – 50%, 10.5min). The residue was concentrated under reduced pressure to remove ACN, and then lyophlization. Title compound 2-[(2S)-4-[7-(8-chloro- 1-naphthyl)-2-[[(2S)-1- methylpyrrolidin-2-yl]methoxy]-6,8-dihydro-5H-pyrido[3,4-d]pyrimidin- 4-yl]-1-(2-fluoroprop-2-enoyl)piperazin-2-yl]acetonitrile (EXAMPLE 7, 24.1 mg, 36.7 umol, 7% yield, 99.1% purity, FA) was obtained as a brown solid. [0434] SFC condition: “AD – 3S_3_5_40_3ML Column: Chiralpak AD – 3 100 × 4.6mm I.D., 3um Mobile phase: methanol (0.05% DEA) in CO2 from 5% to 40% Flow rate: 3mL/min Wavelength: 220nm”. [0435] 1H NMR (400 MHz, Acetic) d = 7.82 (d, J = 8.0 Hz, 1H), 7.69 (d, J = 8.0 Hz, 1H), 7.56 (d, J = 7.6 Hz, 1H), 7.49 (t, J = 7.6 Hz, 1H), 7.41 – 7.30 (m, 2H), 5.58 – 5.25 (m, 2H), 5.17 – 4.59 (m, 4H), 4.57 – 4.28 (m, 3H), 4.24 – 3.78 (m, 4H), 3.67 – 3.13 (m, 7H), 3.08 (br d, J = 2.4 Hz, 3H), 2.98 (br d, J = 6.4 Hz, 1H), 2.83 – 2.61 (m, 1H), 2.45 – 2.29 (m, 1H), 2.24 – 2.08 (m, 3H).
PATENT
US20190144444 https://patents.google.com/patent/US20190144444A1/en
////////

AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
Adagrasib (MRTX849) is an oral, small-molecule KRAS inhibitor developed by Mirati Therapeutics. KRAS mutations are highly common in cancer and account for approximately 85% of all RAS family mutations.5 However, the development of KRAS inhibitors has been challenging due to their high affinity for guanosine triphosphate (GTP) and guanosine diphosphate (GDP), as well as the lack of a clear binding pocket.1 Adagrasib targets KRASG12C, one of the most common KRAS mutations, at the cysteine 12 residue and inhibits KRAS-dependent signalling.2 In a phase I/IB clinical study that included patients with KRASG12C-mutated advanced solid tumors (NCT03785249), adagrasib exhibited anti-tumor activity. The phase II of the same study showed that in patients with KRASG12C-mutated non-small-cell lung cancer (NSCLC), adagrasib was efficient without new safety signals.2,3,6
In February 2022, the FDA accepted a new drug application (NDA) for adagrasib for the treatment of patients with previously treated KRASG12C–positive NSCLC.7 In December 2022, the FDA granted accelerated approval to adagrasib for the treatment of KRASG12C-mutated locally advanced or metastatic NSCLC who have received at least one prior systemic therapy.8,9 Adagrasib joins sotorasib as another KRASG12C inhibitor approved by the FDA.4
Medical uses
Adagrasib is indicated for the treatment of adults with KRAS G12C-mutated locally advanced or metastatic non-small cell lung cancer (NSCLC), as determined by an FDA approved test, who have received at least one prior systemic therapy.[1][2][4]
History
Approval by the US Food and Drug Administration (FDA) was based on KRYSTAL-1, a multicenter, single-arm, open-label clinical trial (NCT03785249) which included participants with locally advanced or metastatic non-small cell lung cancer with KRAS G12C mutations.[2] Efficacy was evaluated in 112 participants whose disease has progressed on or after platinum-based chemotherapy and an immune checkpoint inhibitor, given either concurrently or sequentially.[2]
The FDA granted the application for adagrasib fast-track, breakthrough therapy, and orphan drug designations.[2]
Research
It is undergoing clinical trials.[5][6][7][8][9][10]
References
- ^ Jump up to:a b c d e f g https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/216340s000lbl.pdf
- ^ Jump up to:a b c d e f g h “FDA grants accelerated approval to adagrasib for KRAS G12C-mutated NSC”. U.S. Food and Drug Administration (FDA). 12 December 2022. Retrieved 14 December 2022.
![Public Domain]() This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ^ Jump up to:a b “Mirati Therapeutics Announces U.S. FDA Accelerated Approval of Krazati (adagrasib) as a Targeted Treatment Option for Patients with Locally Advanced or Metastatic Non-Small Cell Lung Cancer (NSCLC) with a KRASG12C Mutation” (Press release). Mirati Therapeutics Inc. 12 December 2022. Retrieved 13 December 2022 – via MultiVu.
- ^ https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/216340Orig1s000ltr.pdf
![Public Domain]() This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ^ Hallin J, Engstrom LD, Hargis L, Calinisan A, Aranda R, Briere DM, et al. (January 2020). “The KRASG12C Inhibitor MRTX849 Provides Insight toward Therapeutic Susceptibility of KRAS-Mutant Cancers in Mouse Models and Patients”. Cancer Discovery. 10 (1): 54–71. doi:10.1158/2159-8290.CD-19-1167. PMC 6954325. PMID 31658955.
- ^ Fell JB, Fischer JP, Baer BR, Blake JF, Bouhana K, Briere DM, et al. (July 2020). “Identification of the Clinical Development Candidate MRTX849, a Covalent KRASG12C Inhibitor for the Treatment of Cancer”. Journal of Medicinal Chemistry. 63 (13): 6679–6693. doi:10.1021/acs.jmedchem.9b02052. PMID 32250617.
- ^ Thein KZ, Biter AB, Hong DS (January 2021). “Therapeutics Targeting Mutant KRAS”. Annual Review of Medicine. 72: 349–364. doi:10.1146/annurev-med-080819-033145. PMID 33138715. S2CID 226242453.
- ^ Christensen JG, Olson P, Briere T, Wiel C, Bergo MO (August 2020). “Targeting Krasg12c -mutant cancer with a mutation-specific inhibitor”. Journal of Internal Medicine. 288 (2): 183–191. doi:10.1111/joim.13057. PMID 32176377.
- ^ Dunnett-Kane V, Nicola P, Blackhall F, Lindsay C (January 2021). “Mechanisms of Resistance to KRASG12C Inhibitors”. Cancers. 13 (1): 151. doi:10.3390/cancers13010151. PMC 7795113. PMID 33466360.
- ^ Jänne PA, Riely GJ, Gadgeel SM, Heist RS, Ou SI, Pacheco JM, et al. (July 2022). “Adagrasib in Non–Small-Cell Lung Cancer Harboring a KRASG12C Mutation”. New England Journal of Medicine. 387 (2): 120–131. doi:10.1056/NEJMoa2204619. PMID 35658005. S2CID 249352736.
External links
- “Adagrasib”. Drug Information Portal. U.S. National Library of Medicine.
- Clinical trial number NCT03785249 for “Phase 1/2 Study of MRTX849 in Patients With Cancer Having a KRAS G12C Mutation KRYSTAL-1” at ClinicalTrials.gov
///////Adagrasib, KRAZATI, FDA 2022, APPROVALS 2022, MRTX-849, MRTX849, Mirati Therapeutics
[H][C@@]1(COC2=NC3=C(CCN(C3)C3=CC=CC4=C3C(Cl)=CC=C4)C(=N2)N2CCN(C(=O)C(F)=C)[C@@]([H])(CC#N)C2)CCCN1C
