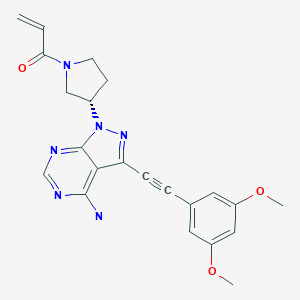

Futibatinib
フチバチニブ
| Formula | C22H22N6O3 |
|---|---|
| CAS | 1448169-71-8 |
| Mol weight | 418.4485 |
2022/9/30 FDA APPROVED, Lytgobi
| Antineoplastic, Receptor tyrosine kinase inhibitor | |
| Disease | Cholangiocarcinoma (FGFR2 gene fusion) |
|---|
1-[(3S)-3-[4-amino-3-[2-(3,5-dimethoxyphenyl)ethynyl]-1H-pyrazolo[3,4-d]pyrimidin-1-yl]-1-pyrrolidinyl]-2-propen-1-one
TAS-120, TAS 120, TAS120; Futibatinib
Futibatinib, also known as TAS-120 is an orally bioavailable inhibitor of the fibroblast growth factor receptor (FGFR) with potential antineoplastic activity. FGFR inhibitor TAS-120 selectively and irreversibly binds to and inhibits FGFR, which may result in the inhibition of both the FGFR-mediated signal transduction pathway and tumor cell proliferation, and increased cell death in FGFR-overexpressing tumor cells. FGFR is a receptor tyrosine kinase essential to tumor cell proliferation, differentiation and survival and its expression is upregulated in many tumor cell types.

SYN
Patent Document 1: International Publication WO 2007/087395 pamphlet
Patent Document 2: International Publication WO 2008/121742 pamphlet
Patent Document 3: International Publication WO 2010/043865 pamphlet
Patent Document 4: International Publication WO 2011/115937 pamphlet
Unlicensed Document 1 : J. Clin. Oncol. 24, 3664-3671 (2006)
Non-licensed Document 2: Mol. Cancer Res. 3, 655-667 (2005)
Non-licensed Document 3: Cancer Res. 70, 2085-2094 (2010)
Non-licensed Document 4: Clin. Cancer Res. 17, 6130-6139 (2011)
Non-licensed Document 5: Nat. Med. 1, 27-31 (1995)
WO2020095452
WO2020096042
WO2020096050
WO2019034075
WO2015008844
WO2015008839
WO2013108809
SYN
US9108973
SYN
Reference Example 1: WXR1
Compound WXR1 was synthesized according to the route reported in patent WO2015008844. 1 H NMR(400MHz, DMSO-d 6 )δ8.40(d,J=3.0Hz,1H),6.93(d,J=2.5Hz,2H),6.74-6.52(m,2H),6.20-6.16( m,1H), 5.74-5.69(m,1H), 5.45-5.61(m,1H), 4.12-3.90(m,2H), 3.90-3.79(m,8H), 2.47-2.30(m,2H). MS m/z: 419.1[M+H] +
PAPER
Bioorg Med Chem, March 2013, Vol.21, No.5, pp.1180-1189
SYN
WO2015008844
PATENT
////////

AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
| Clinical data | |
|---|---|
| Trade names | Lytgobi |
| Other names | TAS-120 |
| License data | US DailyMed: Futibatinib |
| Routes of administration | By mouth |
| Drug class | Antineoplastic |
| ATC code | L01EN04 (WHO) |
| Legal status | |
| Legal status | US: ℞-only [1] |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 1448169-71-8 |
| PubChem CID | 71621331 |
| IUPHAR/BPS | 9786 |
| DrugBank | DB15149 |
| ChemSpider | 58877816 |
| UNII | 4B93MGE4AL |
| KEGG | D11725 |
| ChEMBL | ChEMBL3701238 |
| PDB ligand | TZ0 (PDBe, RCSB PDB) |
| Chemical and physical data | |
| Formula | C22H22N6O3 |
| Molar mass | 418.457 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| showSMILES | |
| showInChI |
Futibatinib, sold under the brand name Lytgobi, is a medication used for the treatment of cholangiocarcinoma (bile duct cancer).[1][2] It is a kinase inhibitor.[1][3] It is taken by mouth.[1]
Futibatinib was approved for medical use in the United States in September 2022.[1][2][4]
Medical uses
Futibatinib is indicated for the treatment of adults with previously treated, unresectable, locally advanced or metastatic intrahepatic cholangiocarcinoma harboring fibroblast growth factor receptor 2 (FGFR2) gene fusions or other rearrangements.[1][2]
Names
Futibatinib is the international nonproprietary name (INN).[5]
References
- ^ Jump up to:a b c d e f “Lytgobi (futibatinib) tablets, for oral use” (PDF). Archived (PDF) from the original on 4 October 2022. Retrieved 4 October 2022.
- ^ Jump up to:a b c https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/214801Orig1s000ltr.pdf Archived 4 October 2022 at the Wayback Machine
![Public Domain]() This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ^ “Lytgobi (Futibatinib) FDA Approval History”. Archived from the original on 4 October 2022. Retrieved 4 October 2022.
- ^ “FDA Approves Taiho’s Lytgobi (futibatinib) Tablets for Previously Treated, Unresectable, Locally Advanced or Metastatic Intrahepatic Cholangiocarcinoma” (Press release). Taiho Oncology. 30 September 2022. Archived from the original on 4 October 2022. Retrieved 4 October 2022 – via PR Newswire.
- ^ World Health Organization (2019). “International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 81”. WHO Drug Information. 33 (1). hdl:10665/330896.
External links
- “Futibatinib”. Drug Information Portal. U.S. National Library of Medicine.
//////////Futibatinib, Lytgobi, FDA 2022, APPROVALS 2022, フチバチニブ , ANTINEOPLASTIC, TAS 120
C=CC(N1C[C@@H](N2N=C(C#CC3=CC(OC)=CC(OC)=C3)C4=C(N)N=CN=C42)CC1)=O
