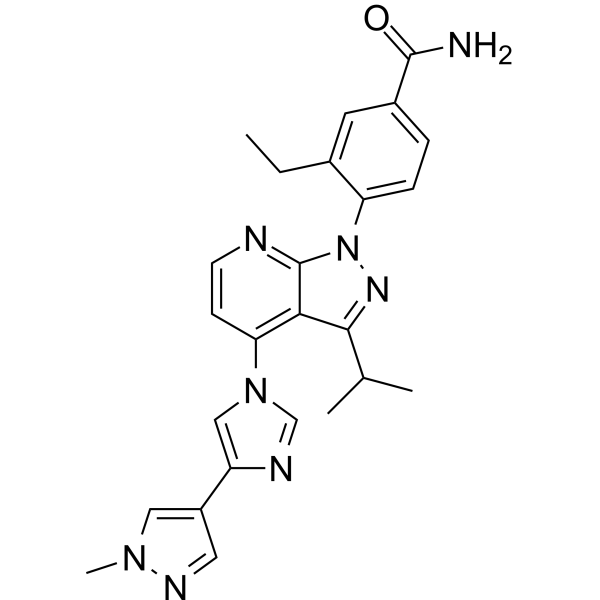
![Benzamide, 3-ethyl-4-[3-(1-methylethyl)-4-[4-(1-methyl-1H-pyrazol-4-yl)-1H-imidazol-1-yl]-1H-pyrazolo[3,4-b]pyridin-1-yl]-.png](http://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?cid=67501411&t=l)
Pimitespib
TAS 116
CAS 1260533-36-5
Antineoplastic, Hsp 90 inhibitor
3-ethyl-4-[4-[4-(1-methylpyrazol-4-yl)imidazol-1-yl]-3-propan-2-ylpyrazolo[3,4-b]pyridin-1-yl]benzamide
Pimitespib (TAS-116) is an oral bioavailable, ATP-competitive, highly specific HSP90α/HSP90β inhibitor (Kis of 34.7 nM and 21.3 nM, respectively) without inhibiting other HSP90 family proteins such as GRP94. Pimitespib demonstrates less ocular toxicity.
| Formula | C25H26N8O |
|---|---|
| CAS | 1260533-36-5 |
| Mol weight | 454.5269 |
JAPAN APPROVED 2022/6/20, ピミテスピブ
| Jeselhy |
Taiho. originator

Pimitespib is a specific inhibitor of heat shock protein 90 (Hsp90) subtypes alpha and beta, with potential antineoplastic and chemo/radiosensitizing activities. Upon oral administration, pimitespib specifically binds to and inhibits the activity of Hsp90 alpha and beta; this results in the proteasomal degradation of oncogenic client proteins, which inhibits client protein dependent-signaling, induces apoptosis, and inhibits the proliferation of cells overexpressing HSP90alpha/beta. Hsp90, a family of molecular chaperone proteins that are upregulated in a variety of tumor cells, plays a key role in the conformational maturation, stability, and function of “client” proteins within the cell,; many of which are involved in signal transduction, cell cycle regulation and apoptosis, including kinases, cell-cycle regulators, transcription factors and hormone receptors. As TAS-116 selectively inhibits cytosolic HSP90alpha and beta only and does not inhibit HSP90 paralogs, such as endoplasmic reticulum GRP94 or mitochondrial TRAP1, this agent may have less off-target toxicity as compared to non-selective HSP90 inhibitors.
Patent
WO2011004610
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2011004610
PATENT
CN108623496
| 3-Ethyl-4-fluorobenzonitrile is an important intermediate for the preparation of a variety of new drugs under development, such as TAS-116, a Phase II clinical drug of Taiho Pharmaceuticals for the treatment of gastrointestinal stromal tumors. |
| |
| Patent WO2005105760 discloses its preparation method. In the method, tetrakis(triphenylphosphine) palladium is used as a catalyst, and 3-bromo-4-fluorobenzonitrile is coupled with tetraethyl tin in a solvent hexamethylphosphoramide for a heating reaction for 15 hours to obtain 3 -Ethyl-4-fluorobenzonitrile. The method uses highly toxic tetraethyl tin, which brings great harm to operators and the environment, and is difficult to carry out industrial production. Meanwhile, the product 3-ethyl-4-fluorobenzonitrile obtained by the preparation method is an oily substance, which is purified by column chromatography with complicated operation, which is unfavorable for industrial production, and the specific purity of the product is not described. |
| |
| Therefore, looking for a new method for preparing 3-ethyl-4-fluorobenzonitrile with cheap and easy-to-obtain raw materials, safe and simple operation, high product purity and low cost suitable for industrial production, which will speed up the research process of related new drugs under development. , it is of great significance to reduce the production cost of related new drugs. |
| Example 1 3-Bromo-4-fluorobenzonitrile |
| |
| 3-Bromo-4-fluorobenzaldehyde (250g, 1.23mol) was dissolved in acetonitrile (1.5L), then hydroxylamine sulfonic acid (67g, 1.48mol) was added, and the reaction was refluxed for 4h. TLC showed that the conversion of the raw materials was complete, and the reaction solution was concentrated. To a small volume, add water (2L) and stir for 30min, cool to 5-10°C and continue stirring for 10min, filter, dissolve the filter cake with methyl tert-butyl ether (1.2L), wash twice with 500ml of water, saturated with 200ml Washed with sodium bicarbonate solution, dried over anhydrous sodium sulfate, filtered, the filtrate was adsorbed with activated carbon (10g), filtered, concentrated under reduced pressure to remove the solvent, added n-heptane (250ml), cooled and stirred in an ice-salt bath for 1h, filtered, reduced Press drying to give 3-bromo-4-fluorobenzonitrile (217 g, 88% yield). 1 H NMR (CDCl 3 ,400MHz):δ7.91(m,1H),7.63(m,1H),7.24(m,1H)。 |
| Example 2 3-Bromo-4-fluorobenzonitrile |
| |
| Add tetrahydrofuran (100ml) to a 250ml reaction flask, add 3-bromo-4-fluorobenzaldehyde (10g, 49.2mmol) and ammonia (40ml) under stirring, add elemental iodine (25g, 98.5mmol) in batches under cooling to 5°C ), then raised to ambient temperature and reacted for 2 to 3 hours, the reaction was completed, the reaction solution was poured into a 10% aqueous solution of sodium sulfite (200g), extracted twice with methyl tert-butyl ether (100ml), dried over anhydrous sodium sulfate , concentrated under reduced pressure to remove the solvent, added n-heptane (20 ml), cooled to 0-10 °C and stirred for 1 h, filtered, and dried under reduced pressure to obtain 3-bromo-4-fluorobenzonitrile (9.6 g, yield: 97.5 %). The NMR spectrum of this compound was determined and was identical to the product of Example 1. |
| Example 3 3-ethyl-4-fluorobenzonitrile |
| |
| 3-Bromo-4-fluorobenzonitrile (200 g, 1 mol) and [1,1-bis(diphenylphosphino)ferrocene]palladium(II) chloride dichloromethane complex (4.08 g, 5mmol) was dissolved in THF (1.2L), 1.0M/L diethylzinc n-hexane solution (600mL, 0.6mol) was added at 40-50°C, and the temperature was raised to 50-60°C for 4-5h. TLC showed The raw materials reacted completely. After the reaction solution was cooled to room temperature, it was added to 5% dilute hydrochloric acid (1 L), the layers were separated, the organic layer was washed twice with 500 ml of water, and then concentrated under reduced pressure to remove the solvent. Then n-hexane (600mL) and activated carbon (20g) were added, refluxed for 0.5h, cooled to room temperature, filtered, then added activated carbon (10g) to the filtrate, refluxed for 0.5h, cooled to room temperature, filtered, and cooled to -50°C to -60°C and filtered, and the filter cake was dried under reduced pressure at 10-20°C to obtain 3-ethyl-4-fluorobenzonitrile (112 g, yield: 75%) as an off-white solid, melting point 23.1-27.4°C. 1 H NMR (CDCl 3 , 400MHz): δ 7.50 (m, 2H), 7.09 (m, 1H), 2.69 (q, J=7.6Hz, 2H), 1.24 (t, 3H, J=7.6Hz), HPLC purity 99.6%. |
| HPLC assay conditions: |
| Chromatographic UV detector: DAD |
| Chromatography pump: 1100 quaternary pump |
| Chromatographic column: Agilent (USA) ZORBAX SB-C184.6×150mm, 5μm PN883975-902 Chromatographic conditions: |
| Mobile Phase A: Water |
| Mobile Phase B: Acetonitrile |
| |
| Injection volume: 5 μL, flow rate: 1.0 mL/min, column temperature: room temperature, detection wavelength: 210 nm. |


Acylation of 2-fluoro-4-iodopyridine with isobutyric anhydride in presence of BuLi and DIEA in THF at -78 °C gives 1-(2-fluoro-4-iodo-3-pyridinyl)-2-methylpropan-1-one ,
This upon cyclization using hydrazine hydrate at 65 °C gives 4-iodo-3-isopropylpyrazolo[3,4-b]pyridine.
N-Protection of intermediate with PMB-Cl in the presence of base NaH in solvent DMF at 0 °C affords 4-iodo-3-isopropyl-1-(4-methoxybenzyl)pyrazolo[3,4-b]pyridine,
This is coupled with 4-(4-imidazolyl)-1-methylpyrazole in the presence of Cu2O, 4,7-dimethoxy-1,10-phenanthroline, Cs2CO3 and PEG-diamine in solvent NMP or DMSO at 130 °C to furnish 4-[4-(4-pyrazolyl)-imidazol-1-yl]pyrazolo[3,4-b]pyridine derivative .
N-Deprotection of PMB-protected pyrazolo[3,4-b]pyridine derivative by using TFA and anisole gives free pyrazolo[3,4-b]pyridine ,
This on condensation with 3-ethyl-4-fluorobenzonitrile in the presence of Cs2CO3 in DMF at 95 °C yields 4-(pyrazolo[3,4-b]pyridin-1-yl)benzonitrile .
Finally, partial hydrolysis of nitrile by means of aqueous NaOH and H2O2 in DMSO/EtOH gives the Pimitespib TAS-116 .
CLIP
https://pubs.acs.org/doi/abs/10.1021/acs.jmedchem.8b01085
J. Med. Chem.2019, 62, 2, 531–551
Publication Date:December 7, 2018
https://doi.org/10.1021/acs.jmedchem.8b0108

The molecular chaperone heat shock protein 90 (HSP90) is a promising target for cancer therapy, as it assists in the stabilization of cancer-related proteins, promoting cancer cell growth, and survival. A novel series of HSP90 inhibitors were discovered by structure–activity relationship (SAR)-based optimization of an initial hit compound 11a having a 4-(4-(quinolin-3-yl)-1H-indol-1-yl)benzamide structure. The pyrazolo[3,4-b]pyridine derivative, 16e (TAS-116), is a selective inhibitor of HSP90α and HSP90β among the HSP90 family proteins and exhibits oral availability in mice. The X-ray cocrystal structure of the 16e analogue 16d demonstrated a unique binding mode at the N-terminal ATP binding site. Oral administration of 16e demonstrated potent antitumor effects in an NCI-H1975 xenograft mouse model without significant body weight loss.
3-Ethyl-4-(3-Isopropyl-4-(4-(1-methyl-1H-Pyrazol-4-yl)-1H-Imidazol-1-yl)-1H-Pyrazolo[3,4-b]pyridin-1-yl)benzamide (16e). Yield 64% (2 steps), white powder. UPLC−MS (ESI) m/z: 454.8 [M + H]+ , tR = 1.19 min. UPLC purity 99.65%. 1 H NMR (400 MHz, CDCl3): δ 1.14 (t, J = 7.5 Hz, 3H), 1.25 (d, J = 7.0 Hz, 6H), 2.62 (q, J = 7.3 Hz, 2H), 3.18 (spt, J = 6.8 Hz, 1H), 3.98 (s, 3H), 5.88 (br s,1H), 6.22 (br s, 1H), 7.13 (d, J = 5.1 Hz, 1H), 7.39 (d, J = 1.1 Hz, 1H), 7.58 (d, J = 8.1 Hz, 1H), 7.78−7.81 (m, 3H), 7.86 (d, J = 1.5 Hz, 1H), 7.96 (d, J = 1.8 Hz, 1H), 8.59 (d, J = 4.7 Hz, 1H). HRMS: calcd for C25H26N8O, 455.2308 [M + H]+ ; found, 455.2311.
PAPER
Journal of Medicinal Chemistry (2021), 64(5), 2669-2677.
PATENT
WO 2016181990
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2016181990
Compound 1 in the present invention is 3-ethyl-4- {3-isopropyl-4- (4- (1-methyl-1H-pyrazol-4-yl) -1H-imidazole-1-yl) -1H-. Pyrazolo [3,4-b] pyridin-1-yl} benzamide (formula below). Compound 1 is known to have HSP90 inhibitory activity and exhibit excellent antitumor activity. Compound 1 can be synthesized based on the production methods described in Patent Documents 1 and 2.
[0013]
[hua 1]
Patent Document 1: International Publication No. 2012/093708
Patent Document 2: International Publication No. 2011/004610
Comparative Example 1 3-Ethyl-4- {3-isopropyl-4- (4- (1-methyl-1H-pyrazole-4-yl) -1H-imidazole-1-yl) -1H-pyrazolo [3, 4-b] Pyridine-1-yl} Synthesis of type I crystals of benzamide
3-Ethyl-4 obtained according to the production method described in International Publication No. 2012/093708 and International Publication No. 2011/004610. -{3-Isopropyl-4- (4- (1-methyl-1H-pyrazole-4-yl) -1H-imidazole-1-yl) -1H-pyrazolo [3,4-b] pyridin-1- A white solid (3.58 g) of yl} benzamide was added to ethanol (7.84 mL) and stirred at room temperature for 2 hours. After sampling, it was washed with ethanol (7.84 mL) and dried under reduced pressure at 70 to 80 ° C. for 20 hours to obtain type I crystals (yield: 2.40 g, yield: 61.2%, purity: 98.21%). rice field.
Further, as shown in FIG. 1, the type I crystal has a diffraction angle (2θ) of 8.1 °, 10.9 °, 12.1 °, 14.0 °, and 14.9 in the powder X-ray diffraction spectrum. °, 16.2 °, 17.7 °, 20.2 °, 21.0 °, 21.5 °, 22.6 °, 24.3 °, 25.4 ° 26.4 °, 27.0 ° , 28.3 °, 30.2 °, 30.9 °, 31.5 °, 32.7 °, 34.7 °, 35.4 ° and 36.6 ° showed characteristic peaks.
[0032]
1H-NMR (DMSO-d 6):δppm 9.35 (1H,d,J=4.88Hz), 8.93 (1H,d,J=1.22Hz), 8.84 (1H,brs), 8.72 (1H,d,J=1.95Hz), 8.70 (1H,s) ,8.63 (1H,d,J=1.22Hz), 8.60 (1H,dd,J=8.29,1.95Hz), 8.46 (1H,s) ,8.25 (1H,d,J=8.29Hz), 8.22 (1H,brs), 8.12 (1H,d,J=4.88Hz), 4.59 (3H,s) ,3.95 (1H,tt,J=6.83,6.83Hz), 3.21 (2H,q,J=7.56Hz), 1.83(6H,d,J=6.83Hz), 1.75 (3H,t,J=7.56Hz):LRMS(ESI)m/z 455[M+H]
PATENT
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2015050235
Synthesis of Test Compound The
following synthesis example compounds (Synthesis Examples 1 to 3) were synthesized according to the method described in WO2011 / 004610.
[0361]
Synthesis Example 1: 4- {3-Isopropyl-4- (4- (1-methyl-1H-pyrazole-4-yl) -1H-imidazol-1-yl) -1H-pyrazolo [3,4-b] pyridine -1-yl} -3-methylbenzamide
[0362]
[Changing 22]
PATENT
WO 2011004610
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2011004610
//////////

AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////

Pimitespib

3-Ethyl-4-{4-[4-(1-methyl-1H-pyrazol-4-yl)-1H-imidazol-1-yl]-3-(propan-2-yl)-1H-pyrazolo[3,4-b]pyridin-1-yl}benzamide
C25H26N8O : 454.53
[1260533-36-5]
//////////Pimitespib, ピミテスピブ, JAPAN 2022, APPROVALS 2022, TAS 116, Jeselhy
O=C(N)C1=CC=C(N2N=C(C(C)C)C3=C(N4C=C(C5=CN(C)N=C5)N=C4)C=CN=C32)C(CC)=C1