

CLOSANTEL
Closantel
57808-65-8
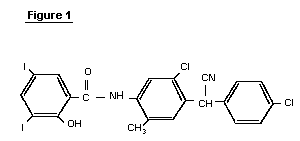
N-(5-chloro-4-((4-chlorophenyl)(cyano)methyl)-2-methylphenyl)-2-hydroxy-3,5-diiodobenzamide
MW 663.1,

Closantel Sodium
CAS NO. 61438-64-0
| FORMULA | C22H13Cl2I2N2O2.Na |
|---|---|
| M. WT | 685.06 |
Closantel
CAS Registry Number: 57808-65-8
CAS Name:N-[5-Chloro-4-[(4-chlorophenyl)cyanomethyl]-2-methylphenyl]-2-hydroxy-3,5-diiodobenzamide
Manufacturers’ Codes: R-31520
Trademarks: Flukiver (Janssen); Seponver (Ethnor)
Molecular Formula: C22H14Cl2I2N2O2, Molecular Weight: 663.07
Percent Composition: C 39.85%, H 2.13%, Cl 10.69%, I 38.28%, N 4.22%, O 4.83%
Literature References: Salicylanilide derivative. Prepn: M. A. C. Janssen, V. K. Sipido, BE839481; eidem,US4005218 (1976, 1977 both to Janssen). Effectiveness against Taenia pisiformis in rabbits: R. A. F. Chevis et al.,Vet. Parasitol.7, 333 (1980); against Ancylostoma caninum: J. Guerrero et al.,J. Parasitol.68, 616 (1983); against Fasciola hepatica in sheep: B. E. Stromberg et al.,ibid.70, 446 (1984). Prolonged effect on Haemonchus contortus in sheep: C. A. Hall et al.,Res. Vet. Sci.31, 104 (1981). Acts by uncoupling oxidative phosphorylation: H. Van den Bossche et al.,Arch. Int. Physiol. Biochim.87, 851 (1979); H. J. Kane et al.,Mol. Biochem. Parasitol.1, 347 (1980).
Properties: Crystals from methanol, mp 217.8°.
Melting point: mp 217.8°
Therap-Cat-Vet: Anthelmintic.
N-{5-chloro-4-[(4-chlorophenyl)(cyano)methyl]-2-methylphenyl}-2-hydroxy-3,5-diiodobenzamide is an aromatic amide resulting from the formal condensation of the carboxy group of 3,5-diiodosalicylic acid with the amino group of aniline substituted at positions 2, 4, and 5 by methyl, (4-chlorophenyl)(cyano)methyl, and methyl groups respectively. It is a nitrile, a member of phenols, an organoiodine compound, a monocarboxylic acid amide, an aromatic amide and a member of monochlorobenzenes.
Closantel is a broad-spectrum antiparasitic agent used against
several species and developmental stages of trematodes, nematodes and
arthropods. The anti-trematode activity of closantel is mainly used
against liver fluke. The anti-nematode and anti-arthropod activity is
especially used against those species which feed on blood or plasma.
The drug is widely used in sheep and cattle and can be used
either parenterally (s.c. or i.m.) or orally for both prophylactic and
therapeutic purposes and is available as drench, bolus and injectable
formulations. Closantel has also been combined with mebendazole and
several other benzimidazoles in drench formulations for sheep and with
levamisole in a bolus for cattle (Marsboom et al., 1989).
Closantel has not been evaluated previously by the Joint FAO/WHO
Expert Committee on Food Additives.
PATENT
https://patents.google.com/patent/CN102180811B/en
Closantel sodium (Closantel Sodium) is a kind of very strong oxidative phosphorylation uncoupler, can suppress the mitochondrial phosphorylation process of polypide, nematode and insect etc. are contacted with blood circulation closely or sucking blood property worm all has and efficiently kills effect, be a kind of broad-spectrum de-worming medicine of efficient, low toxicity, it is huge on market a very large development potentiality.
And 4-chloro-phenyl–(the chloro-4-amino of 2–5-aminomethyl phenyl) cyano group methane is a kind of key intermediate for the synthesis of closantel sodium.But in prior art, the report of the synthetic method of relevant 4-chloro-phenyl–(the chloro-4-amino of 2–5-aminomethyl phenyl) cyano group methane is actually rare, is mainly the iron powder reducing synthetic method.As United States Patent (USP) (US4005218) relates to a kind of with the chloro-α of 4–[the chloro-4-of 2-(hydroxyl imido grpup)-5-methyl-2, the 5-phenylidene] benzyl cyanide (I) is raw material, with excessive iron powder, in ammonium chloride, water and toluene mixing solutions, heating reflux reaction, filter, clean filter cake with a large amount of solvents as tetrahydrofuran (THF) or 4-methyl-2 pentanone, filtrate boils off solvent, then adds the toluene recrystallization to obtain 4-chloro-phenyl–(the chloro-4-amino of 2–5-aminomethyl phenyl) cyano group methane (II).This reaction equation is:
But it is loaded down with trivial details that the shortcoming of the method is operating procedure, the supplementary material consumption is large, and, with producing a large amount of scrap iron powder after iron powder reducing, comparatively thickness, easily comprise product and impurity, and cost recovery is very high, and labour intensity is large, larger to the pollution effect of environment; And product yield and product quality lower.
//////////

AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
PATENTS
CN103054846
WO2015048718
US2015164934
WO2016038035
CN105687172
WO2018013890
WO2018210449
WO2019222349
CN111150725
CN112294793
///////CLOSANTEL, veterinary
CC1=CC(=C(C=C1NC(=O)C2=C(C(=CC(=C2)I)I)O)Cl)C(C#N)C3=CC=C(C=C3)Cl
