
IODOQUINOL
Diiodohydroxyquinoline
- Molecular FormulaC9H5I2NO
- Average mass396.951 Da
- NSC-8704
- SS-578
5,7-Diiodo-8-quinolinol
5,7-Diiodooxine
5,7-diiodoquinolin-8-ol
83-73-8[RN]
8-Hydroxy-5,7-diiodoquinoline
8-Quinolinol, 5,7-diiodo-
дийодогидроксихинолин[Russian][INN]
ثنائي إيودوهيدروكسيكينوليين[Arabic][INN]
双碘喹啉[Chinese][INN]
201-497-9[EINECS]
5,7-Diiodo-8-hydroxyquinoline
IodoquinolCAS Registry Number: 83-73-8
CAS Name: 5,7-Diiodo-8-quinolinol
Additional Names: diiodohydroxyquin; diiodo-oxyquinoline; 5,7-diiodo-8-hydroxyquinoline
Manufacturers’ Codes: SS-578
Trademarks: Diodoquin (Searle); Disoquin; Floraquin (Searle); Dyodin; Dinoleine; Searlequin; Diodoxylin; Rafamebin; Ioquin (Abbott); Direxiode (Delalande); Stanquinate; Yodoxin (Searle); Zoaquin; Enterosept; Embequin (M & B)
Molecular Formula: C9H5I2NO, Molecular Weight: 396.95
Percent Composition: C 27.23%, H 1.27%, I 63.94%, N 3.53%, O 4.03%
Literature References: Prepd by the action of iodine monochloride on 8-hydroxyquinoline: Papesch, Burtner, J. Am. Chem. Soc.58, 1314 (1936); by the action of KIO3 on 8-hydroxyquinoline: Zeifman, C.A.34, 3745. Electrolytic prepn: Brown, Berkowitz, Trans. Electrochem. Soc.75, 385 (1939). See also Claus, DE78880; Passek, DE411050; Matsumura, C.A.21, 1461 (1927); Pirrone, Cherubino, C.A.28, 3073 (1934).Properties: Crystals from xylene. The medicinal grade is a yellowish-brown powder. mp 200-215° (extensive decompn). Almost insol in water. Sparingly sol in alcohol, ether, and acetone; sol in hot pyridine and in hot dioxane.
Melting point: mp 200-215° (extensive decompn)
Therap-Cat: Antiamebic.
Keywords: Antiamebic.
The quinoline derivative diiodohydroxyquinoline (INN), or iodoquinol (USAN), can be used in the treatment of amoebiasis.[1]
It is poorly absorbed from the gastrointestinal tract and is used as a luminal amebicide. It acts by chelation of ferrous ions essential for metabolism.[2]
It was discovered by Adco Co. and introduced as diiodohydroxyquinoline.[3]
Susceptibility of Dientamoeba fragilis has been measured.[4]
Iodoquinol is an amebocide used against Entamoeba histolytica, and it is active against both cyst and trophozoites that are localized in the lumen of the intestine. It is considered the drug of choice for treating asymptomatic or moderate forms of amebiasis. The full mechanism of action is unknown. Iodoquinol is used for diseases caused by moderate intestinal amebiasis.
Diodoquin enhances zinc absorption in the zinc deficiency disorder Acrodermatitis enteropathica, probably because Diodoquin act as a zinc ionophore.[5]
5,7-Diiodo-8-quinolinol Chemical
Originator
Diiodohydroxyquinoline,Adco Co.
Uses
Antiamebic.
Uses
GABA prodrug
Uses
It acts as an amoebicidal and so used in the treatment of amoebiasis, balantidiasis (an infection caused by protozoa).
Indications
Iodoquinol (diiodohydroxyquin, Yodoxin, Moebiquin) is a halogenated 8-hydroxyquinoline derivative whose precise mechanism of action is not known but is thought to involve an inactivation of essential parasite enzymes. Iodoquinol kills the trophozoite forms of E. histolytica, B. coli, B. hominis, and Dientamoeba fragilis.
Iodoquinol is absorbed from the gastrointestinal tract and is excreted in the urine as glucuronide and sulfate conjugates. Most of an orally administered dose is excreted in the feces. Iodoquinol has a plasma half-life of about 12 hours.
Iodoquinol is the drug of choice in the treatment of asymptomatic amebiasis and D. fragilis infections. It is also used in combination with other drugs in the treatment of other forms of amebiasis and as an alternative to tetracycline in the treatment of balantidiasis.
Adverse reactions are related to the iodine content of the drug; the toxicity is often expressed as skin reactions, thyroid enlargement, and interference with thyroid function studies. Headache and diarrhea also occur. Chronic use of clioquinol, a closely related agent, has been linked to a myelitislike illness and to optic atrophy with permanent loss of vision.
Manufacturing Process
5,7-Diiodo-8-quinolinol widely used as an intestinal antiseptic, especially as an antiamebic agent. It is also used topically in other infections and may cause CNS and eye damage. It is known by very many similar trade names worldwide.
0.01 mol 8-oxychinoline and 0.01 mol salicylic acid were dissolved in 500 ml of water and then 0.05 mol potassium iodide was added. The mixture was heated to temperature 90°-100°C. After that 0.01 mol of KIO3 by little tiles was added. The next tile was added after a disappearence of discharging iodine. Then 10 ml 2 N HCl was added. The solid product was fallen, filtered off, washed with hot water and in 0.25 N NaOH dissolved. The solution was filtered and the clear filtrate precipitated with a very little excess of HCl. The product 5,7-diiodo-8-quinolinol was filtered, washed with hot water and dried. MP: 200°-250°C (with decomposition).
brand name
Quinadome (Bayer); Yodoxin (Glenwood).
Therapeutic Function
Antibacterial
Clinical Use
5,7-Diiodo-8-quinolinol, 5,7-diiodo-8-hydroxyquinoline,or diiodohydroxyquin (Yodoxin, Diodoquin, Diquinol) is ayellowish to tan microcrystalline, light-sensitive substancethat is insoluble in water. It is recommended for acute andchronic intestinal amebiasis but is not effective in extraintestinaldisease. Because a relatively high incidence of topicneuropathy has occurred with its use, iodoquinol should notbe used routinely for traveler’s diarrhea.
Safety Profile
Poison by ingestion and intravenous routes. Human systemic effects by ingestion: eye effects. Mutation data reported. When heated to decomposition it emits very toxic fumes of Iand Nox
Chemical Synthesis
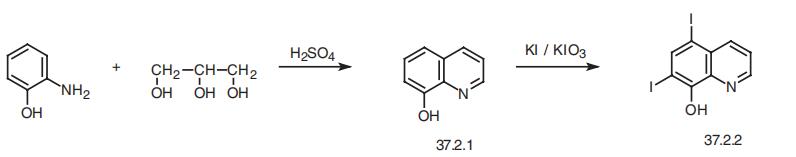
Iodoquinol, 5,7-diiodo-8-quinolinol (37.2.2), is made by iodination of 8-oxyquinoline (37.2.1) using a mixture of potassium iodide/potassium iodate. The initial 8-hydroxyquinolin (37.2.1) is made from 2-aminophenol and glycerol in the presence of sulfuric acid and nitrobenzene (Skraup synthesis).
Purification Methods
It crystallises from xylene and is dried at 70o in a vacuum. [Beilstein 21 II 58.]
5,7-Diiodo-8-quinolinol synthesis
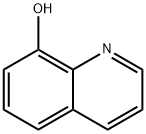
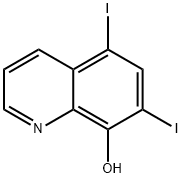
Synthesis of 5,7-Diiodo-8-quinolinol from 8-Hydroxyquinoline
SYN
DE 411050 DOI: 10.1021/ja01298a506

CLIP
Iodoquinol, 5,7-diiodo-8-quinolinol (37.2.2), is made by iodination of 8-oxyquinoline (37.2.1) using a mixture of potassium iodide/potassium iodate. The initial 8-hydroxyquinolin (37.2.1) is made from 2-aminophenol and glycerol in the presence of sulfuric acid and nitrobenzene (Skraup synthesis) [39,40]

Iodoquinol is an amebocide used against E. histolytica, and it is active against both cysts and trophozoites that are localized in the lumen of the intestine. It is considered the drug of choice for treating asymptomatic or moderate forms of amebiasis. The mechanism of action is unknown. Iodoquinol is used for diseases caused by moderate intestinal amebiasis. Synonyms of this drug are diquinol, iodoxin, diiodoquin, amebaquin, and others
39. F. Passek, Ger. Pat. 411.050 (1925). 40. V. Papesch, R.R. Burtner, J. Am. Chem. Soc., 58, 1314 (1936).

AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
References
- ^ Ghaskadbi S, Vaidya VG (March 1989). “In vivo antimutagenic effect of ascorbic acid against mutagenicity of the common antiamebic drug diiodohydroxyquinoline”. Mutat. Res. 222 (3): 219–22. doi:10.1016/0165-1218(89)90137-7. PMID 2493578.
- ^ Nagata, Noriyuki; Marriott, Deborah; Harkness, John; Ellis, John T.; Stark, Damien (2012). “Current treatment options for Dientamoeba fragilis infections”. International Journal for Parasitology: Drugs and Drug Resistance. 2: 204–215. doi:10.1016/j.ijpddr.2012.08.002. ISSN 2211-3207. PMC 3862407. PMID 24533282.
- ^ Publishing, William Andrew (2013-01-15). Pharmaceutical Manufacturing Encyclopedia (3rd ed.). Elsevier Science. p. 1312. ISBN 9780080947266.
- ^ Chan FT, Guan MX, Mackenzie AM, Diaz-Mitoma F (May 1994). “Susceptibility testing of Dientamoeba fragilis ATCC 30948 with iodoquinol, paromomycin, tetracycline, and metronidazole”. Antimicrob. Agents Chemother. 38 (5): 1157–60. doi:10.1128/aac.38.5.1157. PMC 188168. PMID 8067755.
- ^ Aggett, P.J.; Delves, H.T.; Harries, J.T.; Bangham, A.D. (March 1979). “The possible role of Diodoquin as a zinc ionophore in the treatment of acrodermatitis enteropathica”. Biochemical and Biophysical Research Communications. 87 (2): 513–517. doi:10.1016/0006-291X(79)91825-4. PMID 375935.
| Names | |
|---|---|
| Preferred IUPAC name5,7-Diiodoquinolin-8-ol | |
| Other namesDiquinol, iodoxin, diiodoquin, amebaquin | |
| Identifiers | |
| CAS Number | 83-73-8 |
| 3D model (JSmol) | Interactive image |
| ChEBI | CHEBI:5950 |
| ChEMBL | ChEMBL86754 |
| ChemSpider | 3597 |
| ECHA InfoCard | 100.001.362 |
| KEGG | D00581 |
| MeSH | Iodoquinol |
| PubChem CID | 3728 |
| UNII | 63W7IE88K8 |
| CompTox Dashboard (EPA) | DTXSID6023155 |
| showInChI | |
| showSMILES | |
| Properties | |
| Chemical formula | C9H5I2NO |
| Molar mass | 396.951 |
| Pharmacology | |
| ATC code | G01AC01 (WHO) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
 verify (what is ?) verify (what is ?) | |
| Infobox references |
//////////////IODOQUINOL, Diiodohydroxyquinoline, NSC-8704, SS-578
OC1=C2N=CC=CC2=C(I)C=C1I

















