
Zuclopenthixol
Clopenthixol
- Molecular FormulaC22H25ClN2OS
- Average mass400.965 Da
53772-83-1 [RN],
- N05AF05
1-Piperazineethanol, 4-[(3Z)-3-(2-chloro-9H-thioxanthen-9-ylidene)propyl]-
5443
2-{4-[(3Z)-3-(2-Chloro-9H-thioxanthen-9-ylidene)propyl]piperazin-1-yl}ethanol
258-758-5[EINECS]
Z)-Clopenthixol
1-piperazineethanol, 4-(3-(2-chloro-9h-thioxanthen-9-ylidene)propyl)-
1-Piperazineethanol, 4-(3-(2-chlorothioxanthen-9-ylidene)propyl)-
1-Piperazineethanol, 4-[(3Z)-3-(2-chloro-9H-thioxanthen-9-ylidene)propyl]-
- 1-Piperazineethanol, 4-[3-(2-chloro-9H-thioxanthen-9-ylidene)propyl]-, (Z)-
- 4-[(3Z)-3-(2-Chloro-9H-thioxanthen-9-ylidene)propyl]-1-piperazineethanol
- 9H-Thioxanthene, 1-piperazineethanol deriv.
- (Z)-Clopenthixol
- Acuphase
- Cisordinol
- Clopixol
- Clopixol depo
- Zuclopenthixol
- cis-(Z)-Clopenthixol
- cis-Clopenthixol
- α-Clopenthixol
Product Ingredients
| INGREDIENT | UNII | CAS | INCHI KEY |
|---|---|---|---|
| Zuclopenthixol acetate | 349S2ZHF05 | 85721-05-7 | OXAUOBQMCDIVPQ-IOXNKQMXSA-N |
| Zuclopenthixol decanoate | TSS9KIZ5OG | 64053-00-5 | QRUAPADZILXULG-WKIKZPBSSA-N |
| Zuclopenthixol dihydrochloride | 7042692VYN | 58045-23-1 | LPWNZMIBFHMYMX-MHKBYHAFSA-N |
Zuclopenthixol hydrochloride
58045-23-1, MW: 473.8933
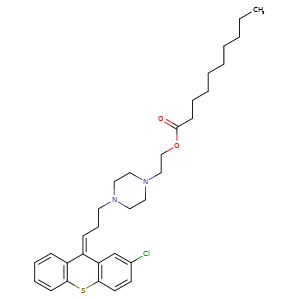
ZUCLOPENTHIXOL DECANOATE, CLOPENTHIXOL DECANOATE, CIS-
64053-00-5, Molecular Formula, C32-H43-Cl-N2-O2-S, Molecular Weight, 555.2227

Zuclopenthixol acetate
85721-05-7, C24H27ClN2O2S, 443.0ClopenthixolCAS Registry Number: 982-24-1
CAS Name: 4-[3-(2-Chloro-9H-thioxanthen-9-ylidene)propyl]-1-piperazineethanol
Additional Names: 2-chloro-9-[3-[4-(2-hydroxyethyl)-1-piperazinyl]propylidene]thiaxanthene
Molecular Formula: C22H25ClN2OS
Molecular Weight: 400.96
Percent Composition: C 65.90%, H 6.28%, Cl 8.84%, N 6.99%, O 3.99%, S 8.00%
Literature References: Thioxanthene neuroleptic. Prepn (configuration not specified): BE585338; P. V. Petersen et al.,US3116291 (1960, 1963 both to Kefalas A/S). Prepn of the pharmacologically active cis-isomer: BE816855; N. Lassen, US3996211 (1974, 1976 both to Kefalas A/S). Pharmacology: Cazzullo, Andreola, Acta Neurol.20, 162 (1965); Weissman, Mod. Probl. Pharmacopsychiatry2, 15 (1969); Moeller Nielsen, ibid. 23. Metabolism: Khan, Acta Pharmacol. Toxicol.27, 202 (1969). HPLC determn of isomers in serum: T. Aaes-Jorgensen, J. Chromatogr.188, 239 (1980). Series of articles on pharmacology and clinical studies: Acta Psychiatr. Scand.64, Suppl. 294, 1-77 (1981).
Properties: Colorless syrup. Sparingly sol in ether. Readily sol in methanol.
Derivative Type: Dihydrochloride
CAS Registry Number: 633-59-0
Manufacturers’ Codes: AY-62021; N-746
Trademarks: Ciatyl (Troponwerke); Sordenac (Lundbeck); Sordinol (Ayerst)
Molecular Formula: C22H25ClN2OS.2HCl
Molecular Weight: 473.89
Percent Composition: C 55.76%, H 5.74%, Cl 22.44%, N 5.91%, O 3.38%, S 6.77%
Properties: Crystals from ethanol, mp 250-260° (dec). Freely sol in water; sparingly sol in alcohol. Practically insol in other organic solvents. LD50 in male mice (mg/kg): 111 i.v. (Lassen).
Melting point: mp 250-260° (dec)
Toxicity data: LD50 in male mice (mg/kg): 111 i.v. (Lassen)
Derivative Type:cis(Z)-Form
CAS Registry Number: 53772-83-1
Additional Names: a-Clopenthixol; zuclopenthixol
Properties: Crystals, mp 84-85°.
Melting point: mp 84-85°
Derivative Type:cis(Z)-Form dihydrochloride
CAS Registry Number: 58045-23-1
Trademarks: Cisordinol (Lundbeck); Clopixol (HMR)
Properties: Crystals, mp 250-260° (dec). LD50 in male mice (mg/kg): 105 i.v. (Lassen).
Melting point: mp 250-260° (dec)
Toxicity data: LD50 in male mice (mg/kg): 105 i.v. (Lassen)
Therap-Cat: Antipsychotic.
Keywords: Antipsychotic; Thioxanthenes.
Zuclopenthixol is an antipsychotic indicated for the management of schizophrenia. The acuphase formulation is indicated for initial treatment of acute psychosis or exacerbation of psychosis, while the depot formulation is best for maintenance.Zuclopenthixol, also known as Zuclopentixol or Zuclopenthixolum, is an antipsychotic agent. Zuclopenthixol is a thioxanthene-based neuroleptic with therapeutic actions similar to the phenothiazine antipsychotics. It is an antagonist at D1 and D2 dopamine receptors. Major brands of zuclopenthixol are Cisordinol, Acuphase, and Clopixol. This drug is a liquid. This compound belongs to the thioxanthenes. These are organic polycyclic compounds containing a thioxanthene moiety, which is an aromatic tricycle derived from xanthene by replacing the oxygen atom with a sulfur atom. Known drug targets of zuclopenthixol include 5-hydroxytryptamine receptor 2A, D(1B) dopamine receptor, D(2) dopamine receptor, D(1A) dopamine receptor, and alpha-1A adrenergic receptor. It is known that zuclopenthixol is metabolized by Cytochrome P450 2D6. Zuclopenthixol was approved for use in Canada in 2011, but is not approved for use in the United States.
Zuclopenthixol (brand names Cisordinol, Clopixol and others), also known as zuclopentixol, is a medication used to treat schizophrenia and other psychoses. It is classed, pharmacologically, as a typical antipsychotic. Chemically it is a thioxanthene. It is the cis–isomer of clopenthixol (Sordinol, Ciatyl).[1] Clopenthixol was introduced in 1961, while zuclopenthixol was introduced in 1978.
Zuclopenthixol is a D1 and D2 antagonist, α1-adrenergic and 5-HT2 antagonist.[2] While it is approved for use in Australia, Canada, Ireland, India, New Zealand, Singapore, South Africa and the UK it is not approved for use in the United States.[3][4]
Medical uses
Available forms
Zuclopenthixol is available in three major preparations:
- As zuclopenthixol decanoate (Clopixol Depot, Cisordinol Depot), it is a long-acting intramuscular injection. Its main use is as a long-acting injection given every two or three weeks to people with schizophrenia who have a poor compliance with medication and suffer frequent relapses of illness.[5] There is some evidence it may be more helpful in managing aggressive behaviour.[6]
- As zuclopenthixol acetate (Clopixol-Acuphase, Cisordinol-Acutard), it is a shorter-acting intramuscular injection used in the acute sedation of psychotic inpatients. The effect peaks at 48–72 hours providing 2–3 days of sedation.[7]
- As zuclopenthixol dihydrochloride (Clopixol, Cisordinol), it is a tablet used in the treatment of schizophrenia in those who are compliant with oral medication.[8]
It is also used in the treatment of acute bipolar mania.
Dosing
As a long-acting injection, zuclopenthixol decanoate comes in a 200 mg and 500 mg ampoule. Doses can vary from 50 mg weekly to the maximum licensed dose of 600 mg weekly. In general, the lowest effective dose to prevent relapse is preferred. The interval may be shorter as a patient starts on the medication before extending to 3 weekly intervals subsequently. The dose should be reviewed and reduced if side effects occur, though in the short-term an anticholinergic medication benztropine may be helpful for tremor and stiffness, while diazepam may be helpful for akathisia. 100 mg of zuclopenthixol decanoate is roughly equivalent to 20 mg of flupentixol decanoate or 12.5 mg of fluphenazine decanoate.
In acutely psychotic and agitated inpatients, 50 – 200 mg of zuclopenthixol acetate may be given for a calming effect over the subsequent three days, with a maximum dose of 400 mg in total to be given. As it is a long-acting medication, care must be taken not to give an excessive dose.
In oral form zuclopenthixol is available in 10, 25 and 40 mg tablets, with a dose range of 20–60 mg daily.
Side effects
Chronic administration of zuclopenthixol (30 mg/kg/day for two years) in rats resulted in small, but significant, increases in the incidence of thyroid parafollicular carcinomas and, in females, of mammary adenocarcinomas and of pancreatic islet cell adenomas and carcinomas. An increase in the incidence of mammary adenocarcinomas is a common finding for D2 antagonists which increase prolactin secretion when administered to rats. An increase in the incidence of pancreatic islet cell tumours has been observed for some other D2 antagonists. The physiological differences between rats and humans with regard to prolactin make the clinical significance of these findings unclear.
Withdrawal syndrome: Abrupt cessation of therapy may cause acute withdrawal symptoms (eg, nausea, vomiting, or insomnia). Symptoms usually begin in 1 to 4 days of withdrawal and subside within 1 to 2 weeks.[1][2]
Other permanent side effects are similar to many other typical antipsychotics, namely extrapyramidal symptoms as a result of dopamine blockade in subcortical areas of the brain. This may result in symptoms similar to those seen in Parkinson’s disease and include a restlessness and inability to sit still known as akathisia, a slow tremor and stiffness of the limbs.[8] Zuclopenthixol is thought to be more sedating than the related flupentixol, though possibly less likely to induce extrapyramidal symptoms than other typical depots.[5] As with other dopamine antagonists, zuclopenthixol may sometimes elevate prolactin levels; this may occasionally result in amenorrhoea or galactorrhoea in severe cases. Neuroleptic malignant syndrome is a rare but potentially fatal side effect. Any unexpected deterioration in mental state with confusion and muscle stiffness should be seen by a physician.
Zuclopenthixol decanoate induces a transient dose-dependent sedation. However, if the patient is switched to maintenance treatment with zuclopenthixol decanoate from oral zuclopenthixol or from i.m. zuclopenthixol acetate the sedation will be no problem. Tolerance to the unspecific sedative effect develops rapidly.[9]
SYN
Journal of the American Chemical Society (2019), 141(6), 2251-2256
https://pubs.acs.org/doi/10.1021/jacs.8b13907
Synthesis of Clopenthixol (4d)
Inside a nitrogen-filled glovebox, an oven-dried glass culture tube (Fischer Scientific part #14- 959-35A), equipped with a magnetic stirring bar, was charged with 2-chloro-9H-thioxanthen-9- one (245 mg, 1.0 mmol, 1 equiv), copper(II) acetate (0.91 mg, 0.0050 mmol, 0.0050 equiv), racBINAP (3.2 mg, 0.0050 mmol, 0.0050 equiv), and THF (1.0 mL). The tube was then fitted tightly with a Teflon-lined blow-out screw cap (Kimble-Chase part #73808-15425). The reaction tube was removed from the glovebox, and the mixture was stirred rapidly for 5 min. A balloon, connected to a 6 mL plastic syringe head, was filled with allene gas until its size was roughly 6 cm in diameter. A needle was attached to the head of the syringe. The reaction tube was evacuated by piercing the septum with a needle connected to a Schlenk line. Immediately after, the allene contained in the balloon was used to refill the reaction tube by piercing the septum with the needle. The balloon decreased to roughly half its original diameter during the refill process. The needle and balloon were left attached, and dimethoxy(methyl)silane (250 uL, 2.0 mmol, 2.0 equiv) was added to the reaction mixture using a 1 mL plastic syringe. The solution was then stirred overnight at rt. At this point, the flask was quickly evacuated by piercing the septum with a needle connected to a Schlenk line, and the headspace was refilled with dry nitrogen. This process was repeated a total of three times. THF (1 mL) solution containing 4e (367 mg, 1.2 mmol, 1.2 equiv), triphenylphosphine (11.5 mg, 0.044 mmol, 0.044 equiv), racDTBM-SEGPHOS (26.8 mg, 0.044 mmol, 0.044 equiv), and copper(II) acetate (3.6 mg, 0.040 mmol, 0.040 equiv) was added to the reaction mixture using a 1 mL plastic syringe. The reaction tube was heated to 40 °C by submersion in an oil bath overnight. After cooling to rt, the cap was removed and 4 M HCl in dioxane was slowly added to the reaction mixture (2.0 mL, WARNING: VIGOROUS HYDROGEN GAS EVOLUTION). The color of the reaction mixture turned to deep red, and after stirring for approximately 30 min, a tan precipitate evolved. After an additional 1 h, diethyl ether (10 mL) was added and the solids collected by filtration (950 mg). By LC/MS analysis, this solid contains mostly 4c (as the hydrochloride) with a trace amount of triphenylphosphine oxide. The entire solid was suspended in dry acetonitrile (1.0 mL) in another dry reaction tube, equipped with a magnetic stirring bar. Potassium carbonate (552 mg, 4 mmol) was added to the tube, which was then capped and placed under a nitrogen atmosphere using a needle connected to a Schlenk line. 2-bromoethanol (142 uL, 2 mmol) was added to the reaction mixture using a glass microsyringe, and the mixture was left to stir overnight at rt. After this time, the cap was removed, and the solution was diluted with water (10 mL). The mixture was extracted with dichloromethane (3 x 10 mL), and the combined organic phases was concentrated with the aid of a rotary evaporator. The mixture was purified by reverse phase preparative HPLC (C18 column, MeCN/water) to yield a 1.1:1 Z/E mixture of 4d as a yellow foamy solid (217 mg, 54% overall yield). The identity of 4d was confirmed by LC/MS analysis against a commercially available standard (Cayman Chemical) and by comparison of 1H NMR to the literature. 13 For further structural confirmation, a portion of 4d was repurified by HPLC to obtain pure (Z)-4d, the biologically active isomer, whose spectra have not been reported in the literature. 1H NMR (400 MHz, CDCl3) δ 7.43 (dd, J = 14.5, 6.2 Hz, 3H), 7.27 (q, J = 8.2 Hz, 4H), 7.17 (d, J = 8.3 Hz, 1H), 5.89 (t, J = 7.1 Hz, 1H), 3.60 (t, J = 5.4 Hz, 2H), 2.62 (t, J = 7.2 Hz, 2H), 2.58–2.35 (m, 12H); 13C NMR (101 MHz, CDCl3) δ 140.2, 135.7, 133.4, 133.2, 132.7, 130.9, 130.4, 128.6, 127.3, 126.9, 126.8, 126.7, 126.2, 125.6, 59.2, 58.3, 57.7, 53.1, 52.8, 27.3.
SYN
Chemical Engineering & Technology (2016), 39(10), 1821-1827.
https://onlinelibrary.wiley.com/doi/10.1002/ceat.201500673
SYN
European Journal of Pharmaceutics and Biopharmaceutics (2012), 82(2), 437-456.
https://www.sciencedirect.com/science/article/abs/pii/S0939641112002263?
SYN
Organic Process Research & Development (2013), 17(9), 1142-1148.
https://pubs.acs.org/doi/10.1021/op400069e
SUN
CN 103214453
https://patents.google.com/patent/CN103214453A/enDiuril ton (Clopenthixol), chemistry 2-chloro-9-[3 ‘ by name-(N ‘-the 2-hydroxyethyl piperazine-N)-allyl group]-thioxanthene, this product is a kind of Thiaxanthene derivative, has significant antipsycholic action and special sedative effect, is particularly useful for the schizophrenia patient.Its activeconstituents is its alpha-isomer, i.e. zuclopenthixol (structural formula is seen Fig. 1); Have the stereotypy effect that anti-Ritalin causes, and the effect of anti-Apomorphine is arranged, this product energy rejection condition avoiding reaction and catalepsy are stronger 10 times than chlorpromazine.A little less than the cholinolytic effect, and antihistamine effect is strong.Zuclopenthixol is applicable to that treatment has psychosis, class Paranoia-illusion type schizophrenia, hebephrenia, the manic and anxiety periodic psychosis of anxiety and illusion symptom; The uneasiness that mental element causes, excitement, psychiatric disorder, the encephalatrophy process, post-traumatic psychosis, the proverb of trembling are absurd etc.Be particularly useful for elderly patients.Recorded the quality standard of zuclopenthixol sheet, zuclopenthixol dihydrochloride, Ciatyl Depot and zuclopenthixol acetic ester etc. in the British Pharmacopoeia, wherein the zuclopenthixol quality standard has stipulated that its content should be 95%-105%.But in actual industrial production, guarantee that zuclopenthixol reaches pharmaceutically acceptable purity, and β-isomer (structural formula is seen Fig. 2) content being limited in 5%, is a very thing of difficulty.About the preparation method of zuclopenthixol, mainly containing of bibliographical information is following several:As if the general separation of having described diuril ton isomer can be undertaken by the fractional crystallization of dihydrochloride among the BE585338A of nineteen fifty-nine application, and still, this separation method yield is extremely low, and complicated operation does not also have actual industrial use.Described the preparation method of diuril ton isomer mixture among the US3116291, wherein alpha-isomer is that the content of zuclopenthixol is 30%-35%.Obtain purer zuclopenthixol by diuril ton alkali being carried out fractional separation in the literary composition with ether organic solvent, but, instructed crystallization to come the purifying zuclopenthixol can not obtain good result, especially in isomer mixture, had under the situation of a large amount of impurity existence by diuril ton alkali.Embodiment 1: the preparation of diuril ton base1) preparation of 2-chloro-9-allyl group-9-thioxanthene alcohol100.00g (0.405mol) 2-chloro-9-thioxanthone is dissolved in the 600mL tetrahydrofuran (THF), 20 ℃ of-30 ℃ of stirrings, add magnesium powder 26g then, iodine 1g, splash into chlorallylene 65g (0.855mol), 40 ℃-50 ℃ are reacted 2h down, and the cooling back drips 20% sodium chloride aqueous solution 1000ml in reaction solution, stir 10min, filter insolubles, use dichloromethane extraction then 2 times, each 500ml, merge organic phase, water 500ml washing is told organic layer, dry after-filtration, filtrate is concentrated except that desolvating, obtain 105.40g2-chloro-9-allyl group-9-thioxanthene alcohol.2) preparation of 2-chloro-9-(2-propenylidene) thioxanthene100.00g (0.346mol) 2-chloro-9-allyl group-9-thioxanthene alcohol is dissolved in the 100ml toluene, solution is heated to 40 ℃, the Acetyl Chloride 98Min. of 1.34g (0.017mol) is dissolved in the diacetyl oxide of 41.19g (0.403mol) and drops in the above-mentioned solution, temperature is controlled at about 40 ℃, dropwise, it is complete until the TLC monitoring reaction that heating makes temperature of reaction rise to 50 ℃ of-55 ℃ of reactions, and concentrating under reduced pressure steams solvent, obtains 94.01g2-chloro-9-(2-propenylidene) thioxanthene.3) preparation of clopenthixol baseN-(2-hydroxyethyl) piperazine of getting 90.00g (0.332mol) 2-chloro-9-(2-propenylidene) thioxanthene and 215.21g (1.65mol) adds in the 1L four-hole boiling flask, stirs and is warming up to 100 ℃ of reactions, and TLC monitors to reacting completely.Vacuum oil pump concentrating under reduced pressure excessive N-(2-hydroxyethyl) piperazine, temperature is controlled at 100 ℃-135 ℃, and oil pump vacuum tightness is at 0.2-1mmHg.Distillation finishes, and adds the benzene of 400ml and the water of 100ml in gained oily matter, and 70 ℃ are stirred 15min, and separatory is used the water washing organic phase of 100ml again, and simultaneous temperature is controlled at 60 ℃-70 ℃, separatory; With organic phase concentrate resistates.This resistates is dissolved in the 300ml methylene dichloride, after add 10% hydrochloric acid soln and transfer pH to 2-3, stirring 10min, separatory, water discard dichloromethane extraction liquid with the dichloromethane extraction of 150ml; Above-mentioned water adds ammoniacal liquor and regulates pH=9-10, extract with methylene dichloride (300ml * 2) after stirring 10min, merge organic phase, use anhydrous sodium sulfate drying, suction filtration, filtrate decompression concentrate 109.32g diuril ton base, isomer proportion α/β is that 45/55 (the HPLC area normalization method: analytical column is 4.6 * 250mmAgilent C18 post, moving phase is acetonitrile: methyl alcohol: phosphoric acid buffer=20: 30: 50, flow velocity are 1mL/min; With this understanding, the retention time of zuclopenthixol is 12min, and the retention time of β-isomer is 16min).Embodiment 2: the preparation of zuclopenthixol Chlorodracylic acid ester 2HCl100.00g (0.250mol) α/β-diuril ton is dissolved in the ethyl acetate of 500ml, under 40 ℃ of conditions, drips the 100ml ethyl acetate that is dissolved with 52.48g (0.30mol) parachlorobenzoyl chloride, dropwise the back back flow reaction, complete until the TLC monitoring reaction.Remove the 300ml solvent under reduced pressure, be cooled to 4 ℃, remove by filter precipitation.Mother liquor is heated to 40 ℃, drips the concentrated hydrochloric acid aqueous solution of 12.50g (0.125mol) 37%, react about 1h after, cool off, have solid to separate out, filter 56.27g zuclopenthixol Chlorodracylic acid ester 2HCl.Purity (HPLC is the same) is 97.11%, and productive rate is 36.82%.Embodiment 3: the preparation of zuclopenthixol2HCl is dissolved in the methanol aqueous solution of 300ml80% with 45.25g (0.074mol) zuclopenthixol Chlorodracylic acid ester, adds the potassium hydroxide of 16.83mol (0.30mol) then.With mixture heating up to 50 ℃, insulation reaction 1h.Underpressure distillation removes and desolvates, and with toluene (200ml * 2) and water extraction, merges organic phase, and concentrating under reduced pressure is removed toluene; Residue obtainedly carry out recrystallization with hexanaphthene, the 25.51g dried crystals.Purity is 99.7%, and productive rate is 86.17%. 1H?NMR(CDCl 3,400MHz),δ:7.10-7.46(7H,m),5.98(1H,t),3.41(2H,t),2.46-2.52(14H,m)。Embodiment 4: the preparation of Ciatyl Depot 2HClThe zuclopenthixol of 50.00g (0.125mol) is dissolved in the methylene dichloride of 500ml, and to wherein dripping 28.60g (0.150mol) decanoyl chloride, back flow reaction is complete to the TLC monitoring reaction after dropwising under the room temperature.Underpressure distillation removes and desolvates, and adds the ethyl acetate of 300ml in residue, drips the ethyl acetate solution that contains hydrogenchloride again, is transferred to 3-4 until pH.After the cooling, filter, vacuum-drying gets 70.63g Ciatyl Depot 2HCl.Productive rate is 90.12%.Embodiment 5: the preparation of Ciatyl DepotThe Ciatyl Depot 2HCl of 60g (0.0957mol) is suspended in the t-butyl methyl ether of 400ml, drips water (250ml) solution of 13.22g (0.0957mol) salt of wormwood, stirring reaction 0.5h.Two are separated, and use 100ml water washing organic phase again, use the anhydrous sodium sulfate drying organic phase, filter, and organic solvent is removed in underpressure distillation, get the 51.29g Ciatyl Depot.Productive rate is 96.58%. 1H?NMR(CDCl 3,400MHz),δ:7.12-7.50(7H,m),5.90(1H,t),4.35(2H,t),3.41(2H,t),2.97(2H,t),2.32-2.57(10H,m),2.06(2H,t),1.64(2H,m),1.30(12H,m),0.88(3H,t)。
SYN
https://patents.google.com/patent/WO2017121755A1/enPreparation of ZU3:9-(3-(4-(2-hydroxyethyl)piperazinyl)propylidene)-thioxanthene

ZU3To a solution of 9-oxothioxanthene (1.0 equiv.) in THF at reflux were added a solution of cyclopropylmagnesium bromide in THF (1.0 equiv.) and stirred during 2 hours. The mixture was cooled down at room temperature and a solution of hydrogen bromide in acetic acid (4 eq.) was added and stirred at room temperature. The reaction mixture was concentrated in vacuo and purified by silica gel column chromatography to obtain 9- (3bromopropylidene)thioxanthene in 30% yield.Then, to a solution of 9-(3bromopropylidene)thioxanthene in acetonitrile at reflux was added N-(2-hydroxyethyl)piperazine (1,5 eq.), potassium iodide (0.1 eq.) and potassium carbonate (3 eq.). The mixture was stirred at reflux then concentrated in vacuo and purified by silica gel column chromatography to afford ZU3 with 98% purity (HPLC). HPLC analysis (BEH C18 type, mobile phase: H20/ acetonitrile (HCOOH 0.1%)) : tR = 1.68 min. Preparation of ZUf:l-(3-(9H-thioxanthen-9-ylidene)propyl)piperidine-4-carboxylic acid), ZU4 (9-(3-(4- (ethylacetate) iperidine)propylidene)-thioxanthene

To a solution of 9-(3-bromopropylidene)thioxanthene in acetonitrile at reflux was added N-ethylacetate piperidine (1,5 eq.), potassium iodide (0.1 eq.) and potassium carbonate (3 eq.). The mixture was stirred at reflux then concentrated in vacuo and purified by silica gel column chromatography to afford ZU4 as a brown oil with a purity of 97% in HPLC analysis HPLC analysis (BEH C18 type, mobile phase: H20/acetonitrile (HCOOH 0.1%)) : tR = 2.07 min.The compound ZU4 was stirred during 2 hours at reflux in a mixture of THF and a solution of NaOH in water. After phase separation, the aqueous layer was extracted twice by diethyl ether. The global organic layer was, then, washed by a saturated solution of NaCl, dried over MgSC^, filtered and concentrated in vacuo to afford ZUf HPLC analysis (BEH CI 8 type, mobile phase: H20/acetonitrile (HCOOH 0.1%)) : tR = 2.24 min.Preparation of ZU5:(Z)-2-(4-(3-(2-chloro-9H-thioxanthen-9-ylidene)propyl)piperazin-l-yl)ethylacetate

To a solution of ZU (4-[3-(2-chloro-9H-thioxanthen-9-ylidene)propyl]-l- piperazineethanol) (leq.) in dichloromethane was added acetic anhydride (1.5 eq.), 4- dimethylaminopyridine (0,1 eq.) and trimethylamine (1 eq.). The mixture was stirred at room temperature and then concentrated in vacuo to afford ZU5 as a yellow oil with 97% of purity (HPLC). HPLC analysis {BEH C18 type, mobile phase: H20/acetonitrile (HCOOH 0.1%)): tR = 2.44 minPreparation of ZUe and ZU6:l-(3-(9H-xanthen-9-ylidene)propyl)piperidine-4-carboxylic acidand eth -(3-(9H-xanthen-9-ylidene)propyl)piperidine-4-carboxylate

To a solution of 9-oxoxanthene (1.0 equiv.) in THF at reflux were added a solution of cyclopropylmagnesium bromide in THF (1.0 equiv.) and stirred during 2 hours. The mixture was cooled down at room temperature and a solution of hydrogen bromide in acetic acid (4 eq.) was added and stirred at room temperature. The reaction mixture was concentrated in vacuo and purified by silica gel column chromatography to obtain 9- (3bromopropylidene)-oxoxanthene.Then, to a solution of 9-(3bromopropylidene)-oxoxanthene in acetonitrile at reflux was added Ethyl 4-piperidinecarboxylate (1,5 eq.), potassium iodide (0.1 eq.) and potassium carbonate (3 eq.). The mixture was stirred at reflux then concentrated in vacuo and purified by silica gel column chromatography to obtain ZU6 ZU6 is then dissolved in a mixture of THF and a solution of NaOH in water. After phase separation, the aqueous layer was extracted twice by diethyl ether. The global organic layer was, then, washed by a saturated solution of NaCl, dried over MgSC^, filtered and concentrated in vacuo to afford ZUe as a white solid with a purity up to 97% in HPLC. Preparation of ZUc:(Z)-2-(4-(3-(2-(trifluoromethyl)-9H-thioxanthen-9-ylidene)propyl)piperazin-l-yl) ethanamine

EtOH, reflux, 2hPurification withHCI buffer

To a solution of ZU1 (2-[4-[3-[2-(trifiuoromethyl)thioxanthen-9- ylidene]propyl]piperidin-l-yl] ethanol) (leq.) in THF was added diethylazodicarboxylate, phtalimide and triphenylphosphine. The solution was stirred at room temperature during 3 hours and then concentrated in vacuo. The crude oil was then dissolved in ethanol, hydrazine was added and the mixture was stirred at reflux during 2 hours. The crude product obtained after concentration was purified via a reversed phase chromatography using HCI as buffer to afford the compound ZUc as an hydrochloride salt (orange solid). [M+H]+ (ESI+) : 434. HPLC analysis (BEH C18 type, mobile phase: H20/acetonitrile (HCOOH 0.1%)): tR = 2.04 min Preparation of ZUd:(Z)-l-(2-fluoroethyl)-4-(3-(2-(trifluoromethyl)-9H-thioxanthen-9-ylidene)propyl) iperazine

To a solution of flupenthixol in dichloromethane was added at -10°C diethylamino sulfur trifluoride. The mixture was then stirred at room temperature. The crude product was purified via a reversed phase chromatography using HC1 as buffer to afford the compound ZUd as a hydrochloride salt (orange solid) with 97% purity in HPLC. HPLC analysis (BEH C18 type, mobile phase: H20/acetonitrile (HCOOH 0.1%)): tR = 3.59 min.Compounds ZU, ZUa, ZUb, ZU1, ZU2The following compounds can be easily found in commerce:

Example 2: Ebselen oxide derivatives

AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
Pharmacology
Pharmacodynamics

Cisordinol 10 mg tablet
Zuclopenthixol antagonises both dopamine D1 and D2 receptors, α1-adrenoceptors and 5-HT2 receptors with a high affinity, but has no affinity for cholinergic muscarine receptors. It weakly antagonises the histamine (H1) receptor but has no α2-adrenoceptor blocking activity[citation needed].
Evidence from in vitro work and clinical sources (i.e. therapeutic drug monitoring databases) suggests that both CYP2D6 and CYP3A4 play important roles in zuclopenthixol metabolism.[11]
Pharmacokinetics
History
Zuclopenthixol was introduced by Lundbeck in 1978.[22]
References
- ^ Sneader, Walter (2005). Drug discovery: a history. New York: Wiley. p. 410. ISBN 0-471-89980-1.
- ^ Pharmacological effects of a specific dopamine D-1 antagonist SCH 23390 in comparison with neuroleptics Life sciences 1984 Apr 16;34(16):1529-40.
- ^ Green, Alan I.; Noordsy, Douglas L.; Brunette, Mary F.; O’Keefe, Christopher (2008). “Substance abuse and schizophrenia: Pharmacotherapeutic intervention”. Journal of Substance Abuse Treatment. 34 (1): 61–71. doi:10.1016/j.jsat.2007.01.008. ISSN 0740-5472. PMC 2930488. PMID 17574793.
- ^ Sweetman, Sean C., ed. (2009). “Anxiolytic Sedatives Hypnotics and Antipsychotics”. Martindale: The complete drug reference (36th ed.). London: Pharmaceutical Press. pp. 1040–1. ISBN 978-0-85369-840-1.
- ^ Jump up to:a b da Silva Freire Coutinho E, Fenton M, Quraishi SN (1999). “Zuclopenthixol decanoate for schizophrenia”. The Cochrane Database of Systematic Reviews. John Wiley and Sons, Ltd. (2): CD001164. doi:10.1002/14651858.CD001164. PMC 7032616. PMID 10796607. Retrieved 2007-06-12.
- ^ Haessler F, Glaser T, Beneke M, Pap AF, Bodenschatz R, Reis O (2007). “Zuclopenthixol in adults with intellectual disabilities and aggressive behaviours”. British Journal of Psychiatry. 190 (5): 447–448. doi:10.1192/bjp.bp.105.016535. PMID 17470962.
- ^ Lundbeck P/L (1991). “Clopixol Acuphase 50 mg/mL Injection Clopixol Acuphase 100 mg / 2 mL Injection”. Lundbeck P/L. Retrieved 2007-06-12.
- ^ Jump up to:a b Bryan, Edward J.; Purcell, Marie Ann; Kumar, Ajit (16 November 2017). “Zuclopenthixol dihydrochloride for schizophrenia”. The Cochrane Database of Systematic Reviews. 2017 (11): CD005474. doi:10.1002/14651858.CD005474.pub2. ISSN 1469-493X. PMC 6486001. PMID 29144549.
- ^ “Summary of Product Characteristics” (PDF).
- ^ Jump up to:a b c d e “TGA eBS – Product and Consumer Medicine Information Licence”.
- ^ Davies SJ, Westin AA, Castberg I, Lewis G, Lennard MS, Taylor S, Spigset O (2010). “Characterisation of zuclopenthixol metabolism by in vitro and therapeutic drug monitoring studies”. Acta Psychiatrica Scandinavica. 122 (6): 445–453. doi:10.1111/j.1600-0447.2010.01619.x. PMID 20946203. S2CID 41869401.
- ^ Parent M, Toussaint C, Gilson H (1983). “Long-term treatment of chronic psychotics with bromperidol decanoate: clinical and pharmacokinetic evaluation”. Current Therapeutic Research. 34 (1): 1–6.
- ^ Jump up to:a b Jørgensen A, Overø KF (1980). “Clopenthixol and flupenthixol depot preparations in outpatient schizophrenics. III. Serum levels”. Acta Psychiatrica Scandinavica. Supplementum. 279: 41–54. doi:10.1111/j.1600-0447.1980.tb07082.x. PMID 6931472.
- ^ Jump up to:a b Reynolds JE (1993). “Anxiolytic sedatives, hypnotics and neuroleptics.”. Martindale: The Extra Pharmacopoeia (30th ed.). London: Pharmaceutical Press. pp. 364–623.
- ^ Ereshefsky L, Saklad SR, Jann MW, Davis CM, Richards A, Seidel DR (May 1984). “Future of depot neuroleptic therapy: pharmacokinetic and pharmacodynamic approaches”. The Journal of Clinical Psychiatry. 45 (5 Pt 2): 50–9. PMID 6143748.
- ^ Jump up to:a b Curry SH, Whelpton R, de Schepper PJ, Vranckx S, Schiff AA (April 1979). “Kinetics of fluphenazine after fluphenazine dihydrochloride, enanthate and decanoate administration to man”. British Journal of Clinical Pharmacology. 7 (4): 325–31. doi:10.1111/j.1365-2125.1979.tb00941.x. PMC 1429660. PMID 444352.
- ^ Young D, Ereshefsky L, Saklad SR, Jann MW, Garcia N (1984). Explaining the pharmacokinetics of fluphenazine through computer simulations. (Abstract.). 19th Annual Midyear Clinical Meeting of the American Society of Hospital Pharmacists. Dallas, Texas.
- ^ Janssen PA, Niemegeers CJ, Schellekens KH, Lenaerts FM, Verbruggen FJ, van Nueten JM, et al. (November 1970). “The pharmacology of fluspirilene (R 6218), a potent, long-acting and injectable neuroleptic drug”. Arzneimittel-Forschung. 20 (11): 1689–98. PMID 4992598.
- ^ Beresford R, Ward A (January 1987). “Haloperidol decanoate. A preliminary review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in psychosis”. Drugs. 33 (1): 31–49. doi:10.2165/00003495-198733010-00002. PMID 3545764.
- ^ Reyntigens AJ, Heykants JJ, Woestenborghs RJ, Gelders YG, Aerts TJ (1982). “Pharmacokinetics of haloperidol decanoate. A 2-year follow-up”. International Pharmacopsychiatry. 17 (4): 238–46. doi:10.1159/000468580. PMID 7185768.
- ^ Larsson M, Axelsson R, Forsman A (1984). “On the pharmacokinetics of perphenazine: a clinical study of perphenazine enanthate and decanoate”. Current Therapeutic Research. 36 (6): 1071–88.
- ^ William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia. Elsevier. pp. 1102–. ISBN 978-0-8155-1856-3.
External links
- Product information for Zuclopenthixol (CLOPIXOL), provided by the Therapeutic Goods Administration — https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2010-PI-05705-3
| Clinical data | |
|---|---|
| Trade names | Clopixol |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category | AU: C |
| Routes of administration | Oral, IM |
| Drug class | Typical antipsychotic |
| ATC code | N05AF05 (WHO) |
| Legal status | |
| Legal status | AU: S4 (Prescription only)UK: POM (Prescription only)In general: ℞ (Prescription only) |
| Pharmacokinetic data | |
| Bioavailability | 49% (oral) |
| Protein binding | 98% |
| Metabolism | Hepatic (CYP2D6 and CYP3A4-mediated) |
| Elimination half-life | 20 hours (oral), 19 days (IM) |
| Excretion | Feces |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 53772-83-1 85721-05-7 (acetate) 64053-00-5 (decanoate) |
| PubChem CID | 5311507 |
| DrugBank | DB01624 |
| ChemSpider | 4470984 |
| UNII | 47ISU063SG |
| KEGG | D03556 |
| ChEBI | CHEBI:51364 |
| ChEMBL | ChEMBL53904 |
| CompTox Dashboard (EPA) | DTXSID3048233 |
| ECHA InfoCard | 100.053.398 |
| Chemical and physical data | |
| Formula | C22H25ClN2OS |
| Molar mass | 400.97 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| showSMILES | |
| showInChI | |
| (what is this?) (verify) |
/////////zuclopenthixol, N05AF05, Clopenthixol, Cisordinol, Clopixol
OCCN1CCN(CC\C=C2\C3=C(SC4=C2C=C(Cl)C=C4)C=CC=C3)CC1
