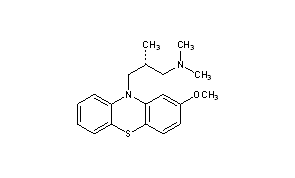
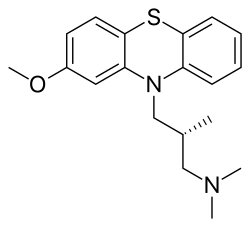
Methotripremazine
- CL 36467
- CL 39743
- N05AA02
- RP 7044
- RP-7044
- SK&F 5116
- XP-03
- XP03
Product Ingredients
| INGREDIENT | UNII | CAS | INCHI KEY |
|---|---|---|---|
| Methotrimeprazine hydrochloride | 42BB1Y2586 | 1236-99-3 | ODLGFPIWRAEFAN-PFEQFJNWSA-N |
| Methotrimeprazine maleate | 5KN5Y9V01K | 7104-38-3 | IFLZPECPTYCEBR-VIEYUMQNSA-N |
Methotrimeprazine
CAS Registry Number: 60-99-1
CAS Name: (bR)-2-Methoxy-N,N,b-trimethyl-10H-phenothiazine-10-propanamine
Additional Names: (-)-10-(3-dimethylamino-2-methylpropyl)-2-methoxyphenothiazine; levomepromazine; 2-methoxytrimeprazine; levomeprazine
Manufacturers’ Codes: RP-7044
Trademarks: Sinogan-Debil; Tisercin (EGYT); Neozine (Rh>e-Poulenc); Nirvan; Nozinan (Rh>e-Poulenc); Levoprome (Lederle)
Molecular Formula: C19H24N2OS
Molecular Weight: 328.47
Percent Composition: C 69.47%, H 7.36%, N 8.53%, O 4.87%, S 9.76%
Literature References: Prepn: Courvoisier et al.,C.R. Seances Soc. Biol. Ses Fil.151, 1378 (1957); Jacob, Robert, US2837518 (1958 to Rhône-Poulenc).Optical Rotatory Power, -17, Conc: 5 g/100mL; Solv: chloroform; Wavlen: 589.3 nm; Temp: 20 °C
Derivative Type: Maleate
CAS Registry Number: 7104-38-3
Trademarks: Minozinan; Milezin (Spofa); Neuractil; Neurocil (Bayer); Sofmin (Dainippon); Veractil
Molecular Formula: C19H24N2OS.C4H4O4
Molecular Weight: 444.54
Percent Composition: C 62.14%, H 6.35%, N 6.30%, O 18.00%, S 7.21%
Properties: Crystals, darkened by light. Dec about 190°. Sparingly sol in water (0.3% at 20°) and in ethanol (0.4%). pH of a 0.3% aq soln is 4.3. The free base is levorotatory: [a]D20 -17° (c = 5 in chloroform).
Optical Rotation: [a]D20 -17° (c = 5 in chloroform)
Therap-Cat: Analgesic.
Keywords: Analgesic (Non-Narcotic).

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////////////////////////////
Methotrimeprazine is a phenothiazine used in the management of psychosis, particular those of schizophrenia, and manic phases of bipolar disorder.
A phenothiazine with pharmacological activity similar to that of both chlorpromazine and promethazine. It has the histamine-antagonist properties of the antihistamines together with central nervous system effects resembling those of chlorpromazine. (From Martindale, The Extra Pharmacopoeia, 30th ed, p604)
Levomepromazine, also known as methotrimeprazine, is a phenothiazine neuroleptic drug. Brand names include Nozinan, Levoprome, Detenler, Hirnamin, Levotomin and Neurocil. It is a low-potency antipsychotic (approximately half as potent as chlorpromazine) with strong analgesic, hypnotic and antiemetic properties that are primarily used in palliative care.[1][2]
Serious side effects include tardive dyskinesia, akathisia, abnormalities in the electrical cycle of the heart, low blood pressure and the potentially fatal neuroleptic malignant syndrome.[1][2]
As is typical of phenothiazine antipsychotics, levomepromazine is a “dirty drug“, that is, it exerts its effects by blocking a variety of receptors, including adrenergic receptors, dopamine receptors, histamine receptors, muscarinic acetylcholine receptors and serotonin receptors.[1][2]
Medical uses
It can be used as an analgesic for moderate to severe pain in non-ambulant patients (the latter being because of its strong sedative effects).[3]
Levomepromazine is also used at lower doses for the treatment of nausea and insomnia.[1]
Levomepromazine is frequently prescribed and valued worldwide in palliative care medicine for its multimodal action, to treat intractable nausea or vomiting, and for severe delirium/agitation in the last days of life. Palliative care physicians will commonly prescribe it orally or via subcutaneous syringe drivers in combination with opioid analgesics such as hydromorphone.[1][2]
Levomepromazine is used for the treatment of psychosis, particularly those of schizophrenia, and manic phases of bipolar disorder. It should only be used with caution in the treatment of agitated depressions, as it can cause akathisia as a side effect, which could worsen the agitation.[1][2] A 2010 systematic review compared the efficacy of levomepromazine with atypical antipsychotic drugs:
Adverse effects
The most common side effect is akathisia.[2] Levomepromazine has prominent sedative and anticholinergic/sympatholytic effects (dry mouth, hypotension, sinus tachycardia, night sweats) and may cause weight gain.[2] These side effects normally preclude prescribing the drug in doses needed for full remission of schizophrenia, so it has to be combined with a more potent antipsychotic.[2] In any case, blood pressure and EKG should be monitored regularly.[2]
A rare but life-threatening side effect is neuroleptic malignant syndrome (NMS).[2] The symptoms of NMS include muscle stiffness, convulsions and fever.[2]
PAPER
Bulletin de la Societe de Pharmacie de Bordeaux (1964), 103(4), 224-30.
The authors define an extn. equil. const., pKe. When a basic mol., A, in an org. solvent (immiscible with water) is shaken with an aq. acid, part of A passes into the aq. phase in the equil. A + H+ .rdblhar. AH+, and Ke and pKe are defined by the equations Ke = [A]org[H+]H2O/[AH+]H2O and pKe = pKa -log ([A]org/[A]H2O), resp. Values of pKe are reported for levomepromazine, properidiazine, thioridazine, chlorpromazine, alimenazine, propiomazine, promethazine, and aminopromazine. Where 2 C atoms sep. the 2 N chain atoms, pKe is of the order of 5, and if 3, the value is near 4.3.
PATENT
JP 40009030
A soln. of 10.5 g. l-3-dimethylamino-2-methylpropanol in xylene is added a suspension of 2.5 g. Na in xylene and a soln. of 18 g. p-tosyl chloride in xylene is dropped in to give l-3-dimethylamino-2-methylpropanol tosylate (I), hydrochloride m. 98-100%. I is treated with 18 g. 2-methoxyphenothiazine and NaNH2 (prepd. from 1.85 g. Na) to give 80% l-3-(2-methoxy-10-phenothiazinyl)-2-methyl-1-dimethylaminopropane, m. 125-6° (hexane). Similarly are prepd. l-3-(3-ethyl-10-phenothiazinyl)-2-methyl-1-dimethylaminopropane (maleate m. 136°) and l-3-(10-phenothiazinyl)-2-methyl-1-dimethylaminopropane (maleate m. 174-5°). The products are tranquilizers.
PATENT
HU 152208
HU 157158
PL 66636
PAPER
Bulletin de la Societe Chimique de France (1968), (8), 3220-2.
Folia medica (1970), 12(1), 88-9
Journal of pharmaceutical sciences (1987), 76(7), 541-4.
SYN
| IN201203390 |
Deprotonation of 2-methoxyphenothiazine by means of KOH in refluxing touene/DMSO, followed by condensation of resulting pottasium salt with N-(3-chloro-2-methylpropyl)-N,N-dimethylamine in refluxing toluene leads to racemic levomepromazine , which upon finally resolution using (-)-dibenzoyl-L-tartaric acid in acetone or using di-p-toluoyl-L-tartaric acid and, optionally, HCOOH in EtOH at 60 °C affords the target levomepromazine

SYN

References
- ^ Jump up to:a b c d e f Brayfield A, ed. (13 December 2013). “Levomepromazine”. Martindale: The Complete Drug Reference. London, UK: Pharmaceutical Press. Retrieved 12 May 2014.
- ^ Jump up to:a b c d e f g h i j k Joint Formulary Committee (2013). British National Formulary (BNF) (65 ed.). London, UK: Pharmaceutical Press. ISBN 978-0-85711-084-8.
- ^ “Levomepromazine”. Farmacotherapeutisch Kompas (in Dutch). Retrieved 5 October 2016.
- ^ Jump up to:a b Sivaraman P, Rattehalli RD, Jayaram MB (October 2010). “Levomepromazine for schizophrenia”. The Cochrane Database of Systematic Reviews. 10 (10): CD007779. doi:10.1002/14651858.CD007779.pub2. PMC 3283151. PMID 20927765.
External links
- “Levomepromazine”. PubChem. National Center for Biotechnology Information.
- NOZINAN – Lévomépromazine Doctissimo Guides des Medicaments
- “Levomepromazine” (PDF). Grampians Palliative Care Team Publication. Victoria, Australia. May 2010. Archived from the original (PDF) on 2011-02-26.
- “Levomepromazine in Palliative Care” (PDF). Scotland, UK. August 2013. Archived from the original (PDF) on 2013-05-22.
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category | Only if clearly needed |
| Routes of administration | Oral, seldom IM |
| Drug class | Typical antipsychotic |
| ATC code | N05AA02 (WHO) |
| Legal status | |
| Legal status | AU: S4 (Prescription only)UK: POM (Prescription only) |
| Pharmacokinetic data | |
| Bioavailability | ~50–60% |
| Metabolism | Hepatic |
| Elimination half-life | ~20 hours |
| Excretion | In feces and urine (metabolites), unchanged drug only 1% |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 60-99-1 7104-38-3 (maleate), 1236-99-3 HCl) |
| PubChem CID | 72287 |
| IUPHAR/BPS | 7603 |
| DrugBank | DB01403 |
| ChemSpider | 65239 |
| UNII | 9G0LAW7ATQ |
| KEGG | D00403 |
| ChEBI | CHEBI:6838 |
| ChEMBL | ChEMBL1764 |
| CompTox Dashboard (EPA) | DTXSID1023289 |
| ECHA InfoCard | 100.000.450 |
| Chemical and physical data | |
| Formula | C19H24N2OS |
| Molar mass | 328.47 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| showSMILES | |
| showInChI | |
| (what is this?) (verify) |
///////////methotripremazine, L 36467, CL 39743, N05AA02, RP 7044, RP-7044, SK&F 5116, XP-03, XP03
O(c2cc1N(c3c(Sc1cc2)cccc3)C[C@H](C)CN(C)C)C
