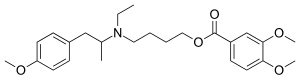
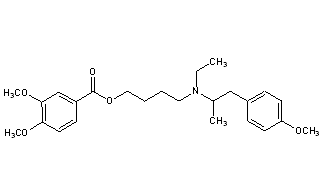
MEBEVERINE
- Molecular FormulaC25H35NO5
- Average mass429.549 Da
3,4-Dimethoxybenzoic Acid 4-[Ethyl[2-(4-methoxyphenyl)-1-methylethyl]amino]butyl Ester
3625-06-7[RN]
222-830-4[EINECS]
3,4-Diméthoxybenzoate de 4-{éthyl[1-(4-méthoxyphényl)-2-propanyl]amino}butyle
мебеверин
ميبيفيرين
美贝维林
- EINECS:222-830-4
- LD50:24 mg/kg (M, i.v.); 995 mg/kg (M, p.o.)
Derivatives
hydrochloride
- Formula:C25H35NO5 • HCl
- MW:466.02 g/mol
- CAS-RN:2753-45-9
- EINECS:220-400-0
- LD50:17.7 mg/kg (R, i.v.); 1540 mg/kg (R, p.o.)
Mebeverine
CAS Registry Number: 3625-06-7
CAS Name: 3,4-Dimethoxybenzoic acid 4-[ethyl[2-(4-methoxyphenyl)-1-methylethyl]amino]butyl ester
Additional Names: veratric acid 4-[ethyl(p-methoxy-a-methylphenethyl)amino]butyl ester;3,4-dimethoxybenzoic acid 4-[ethyl(p-methoxy-a-methylphenethyl)amino]butyl ester;4-[ethyl(p-methoxy-a-methylphenethyl)amino]butyl 3,4-dimethoxybenzoate;4-[N-[2-(p-methoxyphenyl)-1-methylethyl]-N-ethylamino]butyl 3,4-dimethoxybenzoate
Molecular Formula: C25H35NO5
Molecular Weight: 429.55
Percent Composition: C 69.90%, H 8.21%, N 3.26%, O 18.62%
Literature References: Smooth muscle relaxant. Prepn: BE609490C.A.59, 517b (1963) and T. Kralt et al.,DE1126889; eidem,US3265577 (1962, 1962, 1966 to N. V. Philips). Pharmacology: G. Bertaccini et al.,Farmaco Ed. Sci.30, 823 (1975).
Derivative Type: Hydrochloride
CAS Registry Number: 2753-45-9
Trademarks: Colofac (Duphar); Duspatalin (Duphar); Duspatal (Duphar)
Molecular Formula: C25H35NO5.HCl
Molecular Weight: 466.01
Percent Composition: C 64.43%, H 7.79%, N 3.01%, O 17.17%, Cl 7.61%
Properties: Crystals from ethyl methyl ketone, mp 105-107° (Ger. patent); also reported as mp 129-131° (Belg. patent).
Melting point: mp 105-107° (Ger. patent); mp 129-131° (Belg. patent)
Therap-Cat: Antispasmodic.
Keywords: Antispasmodic.

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////////////////////////////
PAT
Indian Pat. Appl., 201841023171

PAPER
Pharma Chemica, 2(2), 366-378; 2010
In a quest of novel antispasmodic agents with antimicrobial properties, the present study describes design and synthesis of novel analogs for veratric acid ester 4-[ethyl-{2-(4- methoxyphenyl)-1-methylethyl} amino] butan-1-ol, an antispasmodic drug which is expected to be a potent antimicrobial agent may be due to the presence of two benzene rings and a secondary or tertiary nitrogen in the basic structural framework of the molecule. The reaction between substituted 2-ethylamino-1-(4’-methoxyphenyl) propane and various haloaryl benzoates derivatives obtained from reaction between different homologs of benzoic acid and dibromoalkanes in a two step process to give corresponding structurally diverse analogs of lead compound has been achieved. The structures of these novel analogs were confirmed by different structure elucidation techniques. All the compounds have been screened for their anti-spasmodic activity and the study extended further to evaluate their sedative, antibacterial and antifungal potency. The novel analogs of lead compound exhibited pronounced antispasmodic activities and also gave encouraging results of antimicrobial and sedative activity as anticipated.
General method of preparation of veratric acid ester 4-[ethyl-{2-(4-methoxyphenyl)-1- methylethyl} amino] butan-1-ol hydrochloride (5) and its analogs (5a-5p) A mixture of compound (3) (149 g, 0.47 mol) and Compound (4) (183 g, 0.95 mol) in ethyl methyl ketone (MEK) was refluxed for a period of 30 h at 75-80oC. The progress of the reaction was monitored by TLC to ensure formation of product and complete conversion of starting. On reaction completion solvent was distilled off and water (750 ml) was added to the reaction mass followed by toluene (300 ml). The resulting solution was cooled to 30oC and stirred for 30 minutes before layer separation. The organic layer was washed further with water (2×100 ml) and dried over sodium sulphate. To the organic layer IPA-HCl (72 g, 20 %) was added till pH is acidic (2-2.5).The product precipitated as solid hydrochloride salt was isolated by filtration and recrystallized from methanol. Yield: 181 g, 82% m.p., 105-107°C.
-C-H stretching (2959-2840), -C=O stretching (1717), -C=C stretching (1605, 1514, 1459), asymmetrical -C-O-C and –C-O stretching (1265-1130), symmetrical -C-O-C stretching (1023)
Chemical Shift ð, 1.25-1.22 ppm (t, 3H, -CH3), 1.57-1.51 (m, 3H, -CH3), 1.90-1.81 (d, 2H, -CH2), 2.16-2.11(m, 2H, – CH2), 2.54-2.25 (m, 1H, -CH), 3.11-3.05 (m, 4H, -CH2), 3.58-3.54 (t, 2H, -CH2), 3.77 (s, 3H, -OCH3), 3.91 (s, 6H, – OCH3), 4.37-4.33 (d, 2H, -CH2), 6.87-6.79 (m, 3H, Ar-H), 7.14-7.12 (d, 2H, Ar-H), 7.52-7.50 (d, 1H, Ar-H), 7.68-7.65 (m, 1H, Ar-H)
Antispasmodic drugs relieve cramps or spasms of the stomach, intestines, and bladder. Antispasmodics are classes (group) of drugs that can help to control some symptoms that arise from the gut, in particular, gut spasm. There are two main types namely “Antimuscarinics” and “Smooth muscle relaxants”. Antispasmodics are commonly used in “Irritable bowel syndrome” (IBS) to help relieve some of the symptoms of IBS such as spasm (colic), bloating and abdominal (stomach) pain and to reduce the motility (movement) of the intestines (gut) [1].
After understanding further the medicinal importance of antispasmodics and their ever increasing demand worldwide, we pursue to undertake the detailed synthetic and pharmacological study of antispasmodics to identify novel candidates as potential drug substances. Our parallel interest also lies on identifying novel antimicrobials since over the years; antibiotics are known to be the major protective agents against bacterial infections. However, the usage of antibiotics and antibacterial chemotherapeutics is becoming more and more restricted in the present age, despite the fact that there exist a large number of antibiotics. This is largely attributed to the emergence of drug-resistant bacteria, which render even some of the most broad spectrum antibiotics ineffective. In addition, most antibiotics have side effects. Thus, it becomes essential to investigate newer drugs with less resistance. Different studies on search of newer antimicrobials and antibacterial have revealed that moderate to remarkable antimicrobial or antibacterial action is present in several compounds, belonging to various pharmacological categories, such as antihistamines [2-4], tranquilizers [5], antihypertensive [6], anti-psychotics [7-11] anti-spasmodic [12] and anti-inflammatory agents [13]. Such compounds, having antibacterial properties in addition to their predesignated pharmacological actions, are termed as non-antibiotics [12]. Many of these compounds possess two or three benzene rings and nitrogen in the secondary or tertiary state in their molecular structure which is expected to be one of the bases for exhibiting antimicrobial potency [14]. Based on this rationale and to pursue our interest to identify newer antispasmodic agents with sedative and antimicrobial properties
[1] M. H. Pittler, E. Ernst, Am. J. Gastroenterol., 1998, 93 (7), 1131–5. [2] S. G. Dastidar, P. K. Saha, B. Sanyamat, A. N. Chakrabarty, J. Appl. Bacteriol., 1976, 41, 209- 214. [3] D. Chattopadyay, S. G. Dastidar, A. Chakrabarty, Arzneimittelforschang, 1988, 38, 869-872. [4] A. Chakrabarty, D. P. Acharya, D. K. Neogi, S. G. Dastidar, Indian J. Med. Res., 1989, 89, 233-237. [5] S. K. Dash, S. G. Dastidar, A. Chakrabarty, Indian J. Exp. Biol., 1977, 15, 324-326. [6] S. G. Dastidar, U. Mondal, S. Niyogi, A. Chakrabarty, Indian J. Med. Res., 1986, 84, 142- 147. [7] J. Molnar, Y. Mandi, J. Kiral, Acta Microbiol Acad Sci Hung., 1976, 23, 45-54. [8] J. E. Kristiansen, Acta Pathol. Microbial Immunol. Scand., 1992, 100 (Suppl. 30), 7-14 [9] S. G. Dastidar, A. Chaudhury, S. Annadurai, M. Mookerjee, A. Chakrabarty, J. Chemother., 1995, 7, 201-206. [10] V. Radhakrishnan, K. Ganguly, M. Ganguly, S. G. Dastidar, A. Chakrabarty, Indian J. Exp. Biol., 1999, 37, 671-675. [11] P. Bourlioux, J. M. Moreaux, W. J. Su, H. Boureau, Acta Pathol. Microbial. Immunol Scand., 1992, 100 (Suppl. 30), 40-43. [12] S. G. Dastidar, A. Chakrabarty, J. Molnar, N. Motohashi, National Institute of Science Communication (NISCOM), New Delhi, 1998, pp. 15. [13] S. Annadurai, S. Basu, S. Ray, S. G. Dastidar, A. C
Mebeverine is a drug used to alleviate some of the symptoms of irritable bowel syndrome. It works by relaxing the muscles in and around the gut.[1]
Medical use
Mebeverine is used to alleviate some of the symptoms of irritable bowel syndrome (IBS) and related conditions; specifically stomach pain and cramps, persistent diarrhoea, and flatulence.[2]
Data from controlled clinical trials have not found a difference from placebo or statistically significant results in the global improvement of IBS.[3][4]
It has not been tested in pregnant women nor in pregnant animals so pregnant women should not take it; it is expressed at low levels in breast milk, while no adverse effects have been reported in infants, breastfeeding women should not take this drug.[1]
Adverse effects
Adverse effects include hypersensitivity reactions and allergic reactions, immune system disorders, skin disorders including hives, oedema and widespread rashes.[2]
Additionally, the following adverse effects have been reported: heartburn, indigestion, tiredness, diarrhoea, constipation, loss of appetite, general malaise, dizziness, insomnia, headache, and decreased pulse rate.[1]
It does not have systemic anticholinergic side effects.[2]
Mebeverine can, on highly rare occasions, cause drug-induced acute angle closure glaucoma.[5]
Mechanism of action
Mebeverine is an anticholinergic but its mechanism of action is not known; it appears to work directly on smooth muscle within the gastrointestinal tract and may have an anaesthetic effect, may affect calcium channels, and may affect muscarinic receptors.[2]
It is metabolized mostly by esterases, and almost completely. The metabolites are excreted in urine.[2]
Mebeverine exists in two enantiomeric forms. The commercially available product is a racemic mixture of them. A study in rats indicates that the two have different pharmacokinetic profiles.[6]
History
It is a second generation papaverine analog, and was first synthesized around the same time as verapamil.[7]
It was first registered in 1965.[8]
Availability
Mebeverine is a generic drug and is available internationally under many brand names.[9]
SYN

Anon., Belgian Patent 609,490 (1962); T. Kralt,
H. O. Moes, A. Lindner and W. J. Asma, German Patent 1,126,889 (1962); Chem. Abstr., 59: 517b
(1963).
SYN

SYN
https://www.sciencedirect.com/science/article/abs/pii/S0731708502000237
References
- ^ Jump up to:a b c “Colofac data sheet” (PDF). New Zealand Medicines and Medical Devices Safety Authority. 14 June 2017. Retrieved 21 July2017.
- ^ Jump up to:a b c d e “Colofac Tablets 135mg – Summary of Product Characteristics (SPC)”. UK Electronic Medicines Compendium. 26 August 2016. Retrieved 21 July 2017.
- ^ Annaházi A, Róka R, Rosztóczy A, Wittmann T (May 2014). “Role of antispasmodics in the treatment of irritable bowel syndrome”. World Journal of Gastroenterology. 20 (20): 6031–43. doi:10.3748/wjg.v20.i20.6031. PMC 4033443. PMID 24876726.
- ^ Darvish-Damavandi M, Nikfar S, Abdollahi M (February 2010). “A systematic review of efficacy and tolerability of mebeverine in irritable bowel syndrome”. World Journal of Gastroenterology. 16(5): 547–53. doi:10.3748/wjg.v16.i5.547. PMC 2816265. PMID 20128021.
- ^ Lachkar Y, Bouassida W (March 2007). “Drug-induced acute angle closure glaucoma”. Current Opinion in Ophthalmology. 18 (2): 129–33. doi:10.1097/ICU.0b013e32808738d5. PMID 17301614. S2CID 30903966.
- ^ Hatami M, Farhadi K, Tukmechi A (August 2012). “Fiber-based liquid-phase micro-extraction of mebeverine enantiomers followed by chiral high-performance liquid chromatography analysis and its application to pharmacokinetics study in rat plasma”. Chirality. 24(8): 634–9. doi:10.1002/chir.22057. PMID 22700279.
- ^ Sneader W (2005). Drug Discovery: A History. John Wiley & Sons. p. 132. ISBN 9780471899792.
- ^ “Mebeverine”. druginfosys. Retrieved 1 February 2015.
- ^ “Mebeverine”. International. drugs.com. Retrieved 1 February2015.
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth |
| ATC code | A03AA04 (WHO) |
| Legal status | |
| Legal status | UK: P (Pharmacy medicines)US: Not approvedIn general: ℞ (Prescription only) |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 3625-06-7 HCl: 2753-45-9 |
| PubChem CID | 4031 |
| ChemSpider | 3891 |
| UNII | 7F80CC3NNVHCl: 15VZ5AL4JN |
| KEGG | D04868 |
| ChEMBL | ChEMBL282121 |
| CompTox Dashboard (EPA) | DTXSID6023238 |
| ECHA InfoCard | 100.020.756 |
| Chemical and physical data | |
| Formula | C25H35NO5 |
| Molar mass | 429.557 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| Chirality | Racemic mixture |
| showSMILES | |
| showInChI | |
| (verify) |
//////////mebeverine, мебеверин ,ميبيفيرين ,美贝维林 , Antispasmodic
