
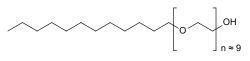

POLIDOCANOL
Synonym: Polidocanol; C12E9, Dodecyl nonaethylene glycol ether, Dodecylnonaglycol, Polidocanol, Polyoxyethylene (9) lauryl ether; trade names: Asclera, Aethoxysklerol and Varithena; Laureth-9; Dodecylnonaoxyethylene glycol monoether
IUPAC/Chemical Name: 3,6,9,12,15,18,21,24,27-nonaoxanonatriacontan-1-ol
3055-99-0
Chemical Formula: C30H62O10
Exact Mass: 582.4343Polidocanol
CAS Registry Number: 9002-92-0
CAS Name: a-Dodecyl-w-hydroxypoly(oxy-1,2-ethanediyl)
Additional Names: polyethylene glycol (9) monododecyl ether; dodecyl alcohol polyoxyethylene ether; hydroxypolyethoxydodecane; laureth 9; polyoxyethylene lauryl ether
Trademarks: Aethoxysklerol (Kreussler); Aetoxisclerol (Dexo); Atlas G-4829 (ICI); Hetoxol L-9 (Heterene Chem.)Line Formula: C12H25(OCH2CH2)nOH
Literature References: Contains an average of nine ethylene oxide units and has an average mol wt ~600. Prepd by reaction of ethylene oxide and dodecyl alcohol: Pertsemlides, Soehring, Arzneim.-Forsch.10, 990 (1960). Toxicology: H. S. Zipf et al.,ibid.7, 162 (1957). Review of clinical experience: P. M. Goldman, J. Dermatol. Surg. Oncol.15, 204-209 (1989).
Properties: Sol in water, ethanol, toluene. Miscible with hot mineral, natural and synthetic oils; with fats and fatty alcohols. LD50 in mice (mg/kg): 1170 orally, 125 i.v. (Zipf).
Toxicity data: LD50 in mice (mg/kg): 1170 orally, 125 i.v. (Zipf)
Use: Solvent; nonionic emulsifier; pharmaceutic aid (surfactant); spermaticide.
Therap-Cat: Anesthetic (topical); antipruritic; sclerosing agent.
Keywords: Anesthetic (Local); Antipruritic; Sclerosing Agent.
| EINECS | 221-284-4 | ||
| CAS No. | 3055-99-0 | Density | 1.007 g/cm3 |
| PSA | 103.30000 | LogP | 4.04900 |
| Solubility | Melting Point | 33-36 °C | |
| Formula | C30H62O10 | Boiling Point | 615.857 °C at 760 mmHg |
| Molecular Weight | 582.43 | Flash Point | 326.259 °C |
Polidocanol is a local anaesthetic and antipruritic component of ointments and bath additives. It relieves itching caused by eczema and dry skin.[1] It has also been used to treat varicose veins,[2] hemangiomas, and vascular malformations.[3] It is formed by the ethoxylation of dodecanol.
Polidocanol is a local anaesthetic and antipruritic component of ointments and bath additives. It relieves itching caused by eczema and dry skin. It is formed by the ethoxylation of dodecanol. The substance is also used as a sclerosant, an irritant injected to treat varicose veins, under the trade names Asclera, Aethoxysklerol and Varithena. Polidocanol causes fibrosis inside varicose veins, occluding the lumen of the vessel, and reducing the appearance of the varicosity. The FDA has approved polidocanol injections for the treatment of small varicose (less than 1 mm in diameter) and reticular veins (1 to 3 mm in diameter). Polidocanol works by damaging the cell lining of blood vessels, causing them to close and eventually be replaced by other types of tissue.

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////////////////////////////
SYN

Yu, Zeqiong; Bo, Shaowei; Wang, Huiyuan; Li, Yu; Yang, Zhigang; Huang, Yongzhuo; Jiang, Zhong-Xing. Application of Monodisperse PEGs in Pharmaceutics: Monodisperse Polidocanols. Molecular Pharmaceutics. Volume 14. Issue 10. Pages 3473-3479. 2017.
SYN 2

Jiang, Zhongxing; Yu, Zeqiong. Process for preparation of monodisperse nona-polyethylene glycol dodecyl alcohol monoether and sulfate. Assignee Wuhan University, Peop. Rep. China. CN 106316802. (2017).
Sclerotherapy
Polidocanol is also used as a sclerosant, an irritant injected to treat varicose veins, under the trade names Asclera, Aethoxysklerol[4] and Varithena.[5] Polidocanol causes fibrosis inside varicose veins, occluding the lumen of the vessel, and reducing the appearance of the varicosity.
The FDA has approved polidocanol injections for the treatment of small varicose (less than 1 mm in diameter) and reticular veins (1 to 3 mm in diameter). Polidocanol works by damaging the cell lining of blood vessels, causing them to close and eventually be replaced by other types of tissue.[6][7] Polidocanol in the form of Varithena injected in the greater saphenous vein can cause the eruption of varicose and spider veins throughout the lower leg. This procedure should be done with caution and with the knowledge that the appearance of the leg may be forever compromised.
Pure polidocanol for pharmaceutical use
On March 30th,2010 the FDA approved Polidocanol under the trade name Asclera. Polidocanol is a sclerosing agent indicated to treat uncomplicated spider veins (varicose veins ≤1 mm in diameter) and uncomplicated reticular veins (varicose veins 1 to 3 mm in diameter) in the lower extremities. Varicose veins develop when the small valves inside the veins no longer work properly, allowing the blood to flow backwards and then pool in the vein.
When injected intravenously, Polidocanol works by locally damaging the endothelium of the blood vessel, causing platelets to aggregate at the site of damage and attach to the venous wall. Eventually, a dense network of platelets, cellular debris and fibrin occludes the vessel, which is then replaced with connective fibrous tissue. As one would expect for this type of molecule and also the mechanism of action, there is believed to be no specific molecular target for Polidocanol.
Polidocanol is a large ‘small molecule’ drug (Molecular Weight of 583 g.mol-1), with a mean half-life of 1.5 hr. Polidocanol is administrated intravenously and the strength of the solution and the volume injected depend on the size and extent of the varicose veins. Thus, the recommended dosage is 0.1 to 0.3 mL for each injection (Asclera 0.5% for spider veins and Asclera 1% for reticular veins) into each varicose vein, and a maximum recommended volume per treatment session of 10 mL.
Polidocanol’s chemical structure is 2-[2-[2-[2-[2-[2-[2-[2-[2-(dodecyloxy)ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethanol. It is a non-ionic detergent, similar to polyethylene glycol (PEG) in structure, consisting of two components, a polar hydrophilic (dodecyl alcohol) and an apolar hydrophobic (polyethylene oxide – the part in brackets in the chemical structure) chain.
References
- ^ “E45 itch relief cream”. netdoctor.co.uk. Retrieved 2007-07-12.
- ^ Star P, Connor DE, Parsi K (April 2018). “Novel developments in foam sclerotherapy: Focus on Varithena® (polidocanol endovenous microfoam) in the management of varicose veins”. Phlebology. 33 (3): 150–162. doi:10.1177/0268355516687864. PMID 28166694.
- ^ Gao Z, Zhang Y, Li W, Shi C (January 2018). “Effectiveness and safety of polidocanol for the treatment of hemangiomas and vascular malformations: A meta-analysis”. Dermatologic Therapy. 31 (1). doi:10.1111/dth.12568. PMID 29082587.
- ^ Sclerotherapy, Laurence Z Rosenberg, MD, eMedicine.com
- ^ “Varithena
![™]() (polidocanol injectable foam) For Intravenous Use. Full Prescribing Information” (PDF). Biocompatibles, Inc. Archived from the original (PDF) on 4 August 2016. Retrieved 1 October 2015.
(polidocanol injectable foam) For Intravenous Use. Full Prescribing Information” (PDF). Biocompatibles, Inc. Archived from the original (PDF) on 4 August 2016. Retrieved 1 October 2015. - ^ Facts and Companies: Varicose Vein Treatment Approved
- ^ “Asclera Full Prescribing Information in Drug Reference Encyclopedia”. Retrieved 2010-04-11.
| Clinical data | |
|---|---|
| Other names | PolydocanolLaureth 9Macrogol lauryl etherLauromacrogolPEG-9 lauryl alcoholPOE-9 lauryl alcoholDodecylpolyethyleneglycoletherHydroxyl polyethoxy dodecaneOxypolyethoxydodecane |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category | Topical: allowed Injection: contraindication in months 1–3 and after week 36 |
| Routes of administration | topical, subcutaneous injection |
| ATC code | C05BB02 (WHO) |
| Legal status | |
| Legal status | OTC (topical), ℞ (injection) |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 9002-92-0 3055-99-0 |
| PubChem CID | 656641 |
| ChemSpider | 570993 |
| UNII | 0AWH8BFG9A |
| KEGG | D01993 |
| ChEMBL | ChEMBL1201751 |
| ECHA InfoCard | 100.019.351 |
| Chemical and physical data | |
| Formula | C30H62O10 |
| Molar mass | 582.816 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| showSMILES | |
| showInChI | |
| (what is this?) (verify) |
////////POLIDOCANOL, Anesthetic , Antipruritic, Sclerosing Agent,
CCCCCCCCCCCCOCCOCCOCCOCCOCCOCCOCCOCCOCCO

 (polidocanol injectable foam) For Intravenous Use. Full Prescribing Information”
(polidocanol injectable foam) For Intravenous Use. Full Prescribing Information”