Bupivacaine
cas 38396-39-3, MF C18H28N2O, Average: 288.4277
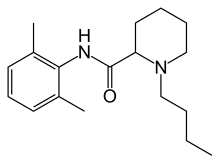
1-butyl-N-(2,6-dimethylphenyl)piperidine-2-carboxamide
- AH 250
- DUR-843
- LAC-43
- SKY 0402
- SKY-0402
- SKY0402
- Win 11318
2-Piperidinecarboxamide, 1-butyl-N-(2,6-dimethylphenyl)-, hydrochloride, hydrate (1:1:1), cas 73360-54-0
Molecular Formula, C18H28N2O.ClH.H2O
Bupivan (Sun) / Carbostesin (AstraZeneca) / Marcain (AstraZeneca) / Marcaina (AstraZeneca) / Posimir (Durect) / Sensorcaine-MPF (Astra Zeneca) / Xaracoll (Innocoll Holdings Limited)
Product Ingredients
| INGREDIENT | UNII | CAS | INCHI KEY |
|---|---|---|---|
| Bupivacaine hydrochloride | 7TQO7W3VT8 | 73360-54-0 | HUCIWBPMHXGLFM-UHFFFAOYSA-N |
| Bupivacaine hydrochloride anhydrous | AKA908P8J1 | 18010-40-7 | SIEYLFHKZGLBNX-UHFFFAOYSA-N |
BupivacaineCAS Registry Number: 2180-92-9
CAS Name: 1-Butyl-N-(2,6-dimethylphenyl)-2-piperidinecarboxamide
Additional Names:dl-1-butyl-2¢,6¢-pipecoloxylidide; 1-n-butyl-2¢,6¢-dimethyl-2-piperidinecarboxanilide; dl-N-n-butylpipecolic acid 2,6-xylidide; 1-butyl-2-(2,6-xylylcarbamoyl)piperidine; dl-1-n-butylpiperidine-2-carboxylic acid 2,6-dimethylanilide
Molecular Formula: C18H28N2O
Molecular Weight: 288.43
Percent Composition: C 74.95%, H 9.78%, N 9.71%, O 5.55%
Literature References: Prepn: B. Ekenstam et al.,Acta Chem. Scand.11, 1183 (1957); B. T. Ekenstam, B. G. Pettersson, US2955111 (1960 to AB Bofors). Resolution of isomers: B. F. Tullar, J. Med. Chem.14, 891 (1971). Stereospecific synthesis: B. Adger et al.,Tetrahedron Lett.37, 6399 (1996).Pharmacology of racemate: F. Henn, R. Brattsand, Acta Anaesthesiol. Scand. Suppl.21, 9 (1966), C.A.66, 17863u (1967); of isomers: F. P. Luduena et al.,Arch. Int. Pharmacodyn.200, 359 (1972). Clinical pharmacokinetics: D. W. Blake et al.,Anaesth. Intensive Care22, 522 (1994). Comprehensive description: T. D. Wilson, Anal. Profiles Drug Subs.19, 59-94 (1990). Review of use in spinal anesthesia: Acta Anaesthesiol. Scand.35, 1-10 (1991). Review of pharmacology and clinical efficacy of levobupivacaine: K. J. McClellan, C. M. Spencer, Drugs56, 355-362 (1998).Properties: mp 107.5-108°. pKa 8.09; also reported as 8.17. Partition coefficient: (oleyl alcohol/water) 1565; (n-heptane/pH 7.4 buffer) 27.5.
Melting point: mp 107.5-108°
pKa: pKa 8.09; also reported as 8.17
Log P: Partition coefficient: (oleyl alcohol/water) 1565; (n-heptane/pH 7.4 buffer) 27.5
Derivative Type: Hydrochloride monohydrate
CAS Registry Number: 14252-80-3
Manufacturers’ Codes: AH-2250; LAC-43
Trademarks: Carbostesin (AstraZeneca); Marcaine (AstraZeneca); Sensorcaine (AstraZeneca)
Molecular Formula: C18H28N2O.HCl.H2O
Molecular Weight: 342.90
Percent Composition: C 63.05%, H 9.11%, N 8.17%, O 9.33%, Cl 10.34%
Properties: White, odorless crystalline powder. mp 258.5°. Slightly sol in acetone, chloroform, ether. Soly (mg/ml): water 40; alcohol 125. LD50 in mice (mg/kg): 7.8 i.v., 82 s.c. (Henn, Brattsand).
Melting point: mp 258.5°
Toxicity data: LD50 in mice (mg/kg): 7.8 i.v., 82 s.c. (Henn, Brattsand)
Derivative Type: (-)-Form
CAS Registry Number: 27262-47-1
Additional Names: Levobupivacaine; (S)-bupivacaine
Properties: Crystals from isopropanol, mp 135-137°. [a]D25 -80.9° (c = 5 in methanol).
Melting point: mp 135-137°
Optical Rotation: [a]D25 -80.9° (c = 5 in methanol)
Derivative Type: (-)-Form hydrochloride
CAS Registry Number: 27262-48-2
Trademarks: Chirocaine (Abbott)
Molecular Formula: C18H28N2O.HCl
Molecular Weight: 324.89
Percent Composition: C 66.54%, H 9.00%, N 8.62%, O 4.92%, Cl 10.91%
Properties: mp 255-257°. [a]D25 -12.3° (c = 2 in water).
Melting point: mp 255-257°
Optical Rotation: [a]D25 -12.3° (c = 2 in water)
Therap-Cat: Anesthetic (local).
Keywords: Anesthetic (Local).
Other Names for this Substance
- 2-Piperidinecarboxamide, 1-butyl-N-(2,6-dimethylphenyl)-, hydrochloride, hydrate (1:1:1)
- 2-Piperidinecarboxamide, 1-butyl-N-(2,6-dimethylphenyl)-, monohydrochloride, monohydrate
- Bupivacaine hydrochloride monohydrate
- Marcain Heavy
- Marcain

(-)-Bupivacaine hydrochloride, Levobupivacaine hydrochloride, Chirocaine
Synthesis Reference
Thuresson, B. and Egner, B.P.H.; U.S. Patent 2,792,399; May 14, 1957; assigned to AB Bofors, Sweden. Thuresson, B. and Pettersson, B.G.; US. Patent 2,955.1 11; October 4,1960; assigned to AB Bofors, Sweden., US2955111
SYN
British Patent 869,978 (1959).

SYN
Bupivacaine, N-2,6-(dimethyl)1-butyl-2-piperidincarboxamide (2.2.7), is chemically similar to mepivacaine and only differs in the replacement of the N-methyl substituent on the piperidine ring with an N-butyl substituent. There are also two suggested methods of synthesis. The first comes from α-picolin-2,6-xylidide (2.2.4). The alkylation of the last with butyl bromide gives the corresponding pyridine salt (2.2.6). Finally, it is reduced by hydrogen using platinum oxide as a catalyst into a piperidine derivative—bupivacaine [13,16].

The other method results directly from the piperidine-2-carboxylic acid chloride, which is reacted with 2,6-dimethylaniline. The resulting amide (2.2.8) is further alkylated with butyl bromide to bupivacaine [17–19].

Like lidocaine and mepivacaine, bupivacaine is used in infiltration, spinal, and epidural anesthesia in blocking nerve transmission. Its most distinctive property is its long-lasting action. It is used for surgical intervention in urology and in lower thoracic surgery from 3 to 5 h in length, and in abdominal surgery lasting from 45 to 60 min. It is used to block the trifacial nerve, the sacral and brachial plexuses, in resetting dislocations, in epidural anesthesia, and during Cesarian sections. The most common synonym for bupivacaine is marcaine.
SYN
3.7 Bupivacaine (21293) and Levobupivacaine (1976)
Bupivacaine (3.1.41) (Marcaine) is a local anesthetic of great potency and long duration that has been widely used for years, but it has cardio and CNS toxic sideeffects. For many years it was nearly the only local anesthetic applicable to almost all kinds of loco-regional anesthetic techniques, and nowadays, in many occasions, it is still the only alternative available [61–64].
Bupivacaine is currently used in racemic form. At high doses, however, the racemate is potentially hazardous due to toxicity problems.
Currently, racemic bupivacaine (3.1.41) is produced from picolinic acid (3.1.38) either by reduction to pipecolic acid (3.1.39) and then, after conversion to corresponding acid chloride (3.1.40) coupling with 2,6-xylidine to give pipecolic acid-2,6-xylidide (3.1.33), or by reducing the pyridyl amide (3.1.43) prepared from picolinic acid chloride (3.1.42) over platinum oxide. The amide intermediate (3.1.33), which can also be used to prepare the anesthetics ropivacaine (3.1.37) and mepivacaine (3.1.31), was transformed to desired bupivacaine (3.1.41) either by direct alkylation using butyl bromide and potassium carbonate or by reductive amination using butyraldehyde [45,59,65–69] (Scheme 3.7).

Enantiomers of bupivacaine can be prepared via diastereomeric salt resolution with tartaric acid or by resolution of the amide (3.1.33) with O,O-dibenzoyl tartaric acid followed by alkylation [47,70].
One of enantiomers, S(–) isomer of the racemic bupivacaine (levobupivacaine), has equal potency but less cardiotoxic and CNS effects in comparison with both R(+) bupivacaine and bupivacaine racemate. The reduced toxicity of levobupivacaine (3.1.48) gives a wider safety margin in clinical practice [71,72].
Stereospecific synthesis of levobupivacaine from (S)-lysine have been proposed (Scheme 3.8).

Treatment of N-CBZ (S)-lysine (3.1.44) with sodium nitrite in acetic acid yields the acetate (3.1.45). The prepared acetate (3.1.45) was then coupled with dimethyl aniline using N,N′-dicyclohexylcarbodiimide to give the amide (3.1.46) in good yield. The acetate group was then converted into the tosylate (3.1.47), which was deprotected and cyclized stereospecifically in one-pot reaction to give the amide (3.1.33) in high yield. Alkylation is easily achieved using an alkyl bromide and K2CO3 without any racemization. Alkylation can also be carried out using butyraldehyde/formic acid although the former is a much simpler process [73] (Scheme 3.8).
SYN
WO 9611181

Levobupivacaine has been obtained by two different ways: 1) The deamination of N-benzoyloxycarbonyl-L-lysine (I) with NaNO2/acetic acid gives 6-acetoxy-2(S)-(benzyl-oxycarbonylamino)hexanoic acid (II), which is amidated with 2,6-dimethylaniline (III) and dicyclohexylcarbodiimide (DCC) to the expected amide (IV). The deacetylation of (IV) with K2CO3 in methanol affords compound (V), which is tosylated as usual with tosyl chloride giving intermediate (VI), which is stereospecifically cyclized by means of K2CO3 in ethanol yielding N-(2,6-dimethyl-phenyl)piperidine-2 (S)-carboxamide (VII). Finally, this compound is alkylated with butyl bromide and K2CO3 or by reductoalkylation with butyraldehyde. 2) The amidation of piperidine-2-carboxylic acid (VIII) with 2,6-dimethylaniline (III) by means of SOCl2 in toluene gives the corresponding amide (IX), which is alkylated with butyl bromide as before yielding racemic bupivacaine (X) (3). This compound is then submitted to optical resolution by treatment with (S,S)-(?-tartaric acid followed by crystallization of the resulting tartrate and acidification with HCl in isopropanol.
SYN
| Org Process Res Dev 2000,4(6),530 |

Improved yield in the synthesis of levobupivacaine. An improved yield in the synthesis of levobupivacaine can be obtained by recovering the unwanted (R)-enantiomer side product in the optical resolution of the racemic bupivacaine. The treatment of (R)-(I) with refluxing propionic acid causes its racemization, yielding racemic-(I) (bupivacaine), which is then submitted to a new optical resolution process using dibenzoyl-L-tartaric acid.
Literatures:
Acta Chemica Scandinavica (1947-1973), , vol. 11, p. 1183,1184
Literatures:
BRIDGE PHARMA, INC. Patent: WO2008/88756 A1, 2008 ; Location in patent: Page/Page column 30-31 ;
Yield: ~94%
nmr






join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////////////////////////////
Bupivacaine is a local anesthetic used in a wide variety of superficial and invasive procedures.
Bupivacaine, marketed under the brand name Marcaine among others, is a medication used to decrease feeling in a specific area.[4] In nerve blocks, it is injected around a nerve that supplies the area, or into the spinal canal’s epidural space.[4] It is available mixed with a small amount of epinephrine to increase the duration of its action.[4] It typically begins working within 15 minutes and lasts for 2 to 8 hours.[4][5]
Possible side effects include sleepiness, muscle twitching, ringing in the ears, changes in vision, low blood pressure, and an irregular heart rate.[4] Concerns exist that injecting it into a joint can cause problems with the cartilage.[4] Concentrated bupivacaine is not recommended for epidural freezing.[4] Epidural freezing may also increase the length of labor.[4] It is a local anaesthetic of the amide group.[4]
Bupivacaine was discovered in 1957.[6] It is on the World Health Organization’s List of Essential Medicines.[7] Bupivacaine is available as a generic medication.[4][8] An implantable formulation of bupivacaine (Xaracoll) was approved for medical use in the United States in August 2020.[9][10][11]
Medical uses
Bupivacaine is indicated for local infiltration, peripheral nerve block, sympathetic nerve block, and epidural and caudal blocks. It is sometimes used in combination with epinephrine to prevent systemic absorption and extend the duration of action. The 0.75% (most concentrated) formulation is used in retrobulbar block.[12] It is the most commonly used local anesthetic in epidural anesthesia during labor, as well as in postoperative pain management.[13] Liposomal formulations of bupivacaine (brand name EXPAREL) have shown to be more effective in providing pain relief than plain solutions of bupivacaine.[14][15]
The fixed-dose combination of bupivacaine with Type I collagen (brand name Xaracoll) is indicated for acute postsurgical analgesia (pain relief) for up to 24 hours in adults following open inguinal hernia repair.[10][11]
Bupivacaine (Posimir) is indicated in adults for administration into the subacromial space under direct arthroscopic visualization to produce post-surgical analgesia for up to 72 hours following arthroscopic subacromial decompression.[16][17]
Contraindications
Bupivacaine is contraindicated in patients with known hypersensitivity reactions to bupivacaine or amino-amide anesthetics. It is also contraindicated in obstetrical paracervical blocks and intravenous regional anaesthesia (Bier block) because of potential risk of tourniquet failure and systemic absorption of the drug and subsequent cardiac arrest. The 0.75% formulation is contraindicated in epidural anesthesia during labor because of the association with refractory cardiac arrest.[18]
Adverse effects
Compared to other local anaesthetics, bupivacaine is markedly cardiotoxic.[19] However, adverse drug reactions (ADRs) are rare when it is administered correctly. Most ADRs are caused by accelerated absorption from the injection site, unintentional intravascular injection, or slow metabolic degradation. However, allergic reactions can rarely occur.[18]
Clinically significant adverse events result from systemic absorption of bupivacaine and primarily involve the central nervous system (CNS) and cardiovascular system. CNS effects typically occur at lower blood plasma concentrations. Initially, cortical inhibitory pathways are selectively inhibited, causing symptoms of neuronal excitation. At higher plasma concentrations, both inhibitory and excitatory pathways are inhibited, causing CNS depression and potentially coma. Higher plasma concentrations also lead to cardiovascular effects, though cardiovascular collapse may also occur with low concentrations.[20] Adverse CNS effects may indicate impending cardiotoxicity and should be carefully monitored.[18]
- CNS: circumoral numbness, facial tingling, vertigo, tinnitus, restlessness, anxiety, dizziness, seizure, coma
- Cardiovascular: hypotension, arrhythmia, bradycardia, heart block, cardiac arrest[13][18]
Toxicity can also occur in the setting of subarachnoid injection during high spinal anesthesia. These effects include: paresthesia, paralysis, apnea, hypoventilation, fecal incontinence, and urinary incontinence. Additionally, bupivacaine can cause chondrolysis after continuous infusion into a joint space.[18]
Bupivacaine has caused several deaths when the epidural anaesthetic has been administered intravenously accidentally.[21]
Treatment of overdose
Further information: Lipid rescue
Animal evidence[22][23] indicates intralipid, a commonly available intravenous lipid emulsion, can be effective in treating severe cardiotoxicity secondary to local anaesthetic overdose, and human case reports of successful use in this way.[24][25] Plans to publicize this treatment more widely have been published.[26]
Pregnancy and lactation
Bupivacaine crosses the placenta and is a pregnancy category C drug. However, it is approved for use at term in obstetrical anesthesia. Bupivacaine is excreted in breast milk. Risks of discontinuing breast feeding versus discontinuing bupivacaine should be discussed with the patient.[18]
Postarthroscopic glenohumeral chondrolysis
Bupivacaine is toxic to cartilage and its intra-articular infusions may lead to postarthroscopic glenohumeral chondrolysis.[27]
Pharmacology
Pharmacodynamics
Bupivacaine binds to the intracellular portion of voltage-gated sodium channels and blocks sodium influx into nerve cells, which prevents depolarization. Without depolarization, no initiation or conduction of a pain signal can occur.
Pharmacokinetics
The rate of systemic absorption of bupivacaine and other local anesthetics is dependent upon the dose and concentration of drug administered, the route of administration, the vascularity of the administration site, and the presence or absence of epinephrine in the preparation.[28]
- Onset of action (route and dose-dependent): 1-17 min
- Duration of action (route and dose-dependent): 2-9 hr
- Half life: neonates, 8.1 hr, adults: 2.7 hr
- Time to peak plasma concentration (for peripheral, epidural, or caudal block): 30-45 min
- Protein binding: about 95%
- Metabolism: hepatic
- Excretion: renal (6% unchanged)[18]
Chemical structure
Like lidocaine, bupivacaine is an amino-amide anesthetic; the aromatic head and the hydrocarbon chain are linked by an amide bond rather than an ester as in earlier local anesthetics. As a result, the amino-amide anesthetics are more stable and less likely to cause allergic reactions. Unlike lidocaine, the terminal amino portion of bupivacaine (as well as mepivacaine, ropivacaine, and levobupivacaine) is contained within a piperidine ring; these agents are known as pipecholyl xylidines.[13]
Society and culture
Legal status
On 17 September 2020, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Exparel, intended for the treatment of post-operative pain.[29] The applicant for this medicinal product is Pacira Ireland Limited.[29] Exparel liposomal was approved for medical use in the European Union in November 2020.[30]
Economics
Bupivacaine is available as a generic medication.[4][8]
Research
Levobupivacaine is the (S)-(–)-enantiomer of bupivacaine, with a longer duration of action, producing less vasodilation. Durect Corporation is developing a biodegradable, controlled-release drug delivery system for after surgery. It has currently[when?] completed a phase-III clinical trial.[31]
References
- ^ “Bupivacaine Use During Pregnancy”. Drugs.com. 13 April 2020. Retrieved 21 September 2020.
- ^ “Marcaine- bupivacaine hydrochloride injection, solution Marcaine with epinephrine- bupivacaine hydrochloride and epinephrine bitartrate injection, solution”. DailyMed. Retrieved 13 February2021.
- ^ “Sensorcaine MPF- bupivacaine hydrochloride injection, solution”. DailyMed. Retrieved 13 February 2021.
- ^ Jump up to:a b c d e f g h i j k l m n “Bupivacaine Hydrochloride”. The American Society of Health-System Pharmacists. Archived from the original on 2015-06-30. Retrieved August 1, 2015.
- ^ Jump up to:a b Whimster, David Skinner (1997). Cambridge textbook of accident and emergency medicine. Cambridge: Cambridge University Press. p. 194. ISBN 9780521433792. Archived from the original on 2015-10-05.
- ^ Egan, Talmage D. (2013). Pharmacology and physiology for anesthesia : foundations and clinical application. Philadelphia, PA: Elsevier/Saunders. p. 291. ISBN 9781437716795. Archivedfrom the original on 2016-05-12.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06.
- ^ Jump up to:a b Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 22. ISBN 9781284057560.
- ^ “Xaracoll: FDA-Approved Drugs”. U.S. Food and Drug Administration (FDA). Retrieved 2 September 2020.
- ^ Jump up to:a b “FDA approval letter” (PDF). U.S. Food and Drug Administration (FDA). 28 August 2020. Retrieved 2 September2020.
![Public Domain]() This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ^ Jump up to:a b “FDA Approves Xaracoll (bupivacaine HCl) Implant, a Non-opioid, Drug-device Treatment Option for Acute Postsurgical Pain Relief for up to 24 Hours Following Open Inguinal Hernia Repair in Adults” (Press release). Innocoll Pharmaceuticals. 31 August 2020. Retrieved 2 September 2020 – via PR Newswire.
- ^ Lexicomp. “Bupivacaine (Lexi-Drugs)”. Archived from the original on 2014-04-10. Retrieved 20 April 2014.
- ^ Jump up to:a b c Miller, Ronald D. (November 2, 2006). Basics of Anesthesia. Churchill Livingstone.
- ^ Ma, Ting-Ting, et al. (2017). “Liposomal bupivacaine versus traditional bupivacaine for pain control after total hip arthroplasty: A meta-analysis”. Medicinevol. 96 (96, 25 (2017): e7190): e7190. doi:10.1097/MD.0000000000007190. PMC 5484209. PMID 28640101.
- ^ Mont, M. A., Beaver, W. B., Dysart, S. H., Barrington, J. W., & Gaizo, D. J. (2018). “Local Infiltration Analgesia With Liposomal Bupivacaine Improves Pain Scores and Reduces Opioid Use After Total Knee Arthroplasty: Results of a Randomized Controlled Trial”. The Journal of Arthroplasty. 33 (1): 33(1), 90–96. doi:10.1016/j.arth.2017.07.024. PMID 28802777.
- ^https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2021/204803Orig1s000ltr.pdf
- ^ “Durect Corporation Announces U.S. FDA Approval of Posimir For Post-Surgical Pain Reduction for up to 72 Hours Following Arthroscopic Subacromial Decompression” (Press release). Durect Corporation. 2 February 2021. Retrieved 13 February 2021 – via PR Newswire.
- ^ Jump up to:a b c d e f g “Bupivacaine (Lexi-Drugs)”. Archived from the original on 2014-04-10. Retrieved 20 April 2014.
- ^ de La Coussaye, J. E.; Eledjam, J. J.; Brugada, J.; Sassine, A. (1993). “[Cardiotoxicity of local anesthetics]”. Cahiers d’Anesthésiologie. 41 (6): 589–598. ISSN 0007-9685. PMID 8287299.
- ^ Australian Medicines Handbook. Adelaide. 2006. ISBN 978-0-9757919-2-9.
- ^ ABS-CBN Interactive: Filipino nurse dies in UK due to wrong use of anaesthetic
- ^ Weinberg, GL; VadeBoncouer, T; Ramaraju, GA; Garcia-Amaro, MF; Cwik, MJ. (1998). “Pretreatment or resuscitation with a lipid infusion shifts the dose-response to bupivacaine-induced asystole in rats”. Anesthesiology. 88 (4): 1071–5. doi:10.1097/00000542-199804000-00028. PMID 9579517. S2CID 1661916.
- ^ Weinberg, G; Ripper, R; Feinstein, DL; Hoffman, W. (2003). “Lipid emulsion infusion rescues dogs from bupivacaine-induced cardiac toxicity”. Regional Anesthesia and Pain Medicine. 28 (3): 198–202. doi:10.1053/rapm.2003.50041. PMID 12772136. S2CID 6247454.
- ^ Rosenblatt, MA; Abel, M; Fischer, GW; Itzkovich, CJ; Eisenkraft, JB (July 2006). “Successful use of a 20% lipid emulsion to resuscitate a patient after a presumed bupivacaine-related cardiac arrest”. Anesthesiology. 105 (1): 217–8. doi:10.1097/00000542-200607000-00033. PMID 16810015.
- ^ Litz, RJ; Popp, M; Stehr, S N; Koch, T. (2006). “Successful resuscitation of a patient with ropivacaine-induced asystole after axillary plexus block using lipid infusion”. Anaesthesia. 61 (8): 800–1. doi:10.1111/j.1365-2044.2006.04740.x. PMID 16867094. S2CID 43125067.
- ^ Picard, J; Meek, T (February 2006). “Lipid emulsion to treat overdose of local anaesthetic: the gift of the glob”. Anaesthesia. 61(2): 107–9. doi:10.1111/j.1365-2044.2005.04494.x. PMID 16430560. S2CID 29843241.
- ^ Gulihar, Abhinav; Robati, Shibby; Twaij, Haider; Salih, Alan; Taylor, Grahame J.S. (December 2015). “Articular cartilage and local anaesthetic: A systematic review of the current literature”. Journal of Orthopaedics. 12 (Suppl 2): S200–S210. doi:10.1016/j.jor.2015.10.005. PMC 4796530. PMID 27047224.
- ^ “bupivacaine hydrochloride (Bupivacaine Hydrochloride) injection, solution”. FDA. Archived from the original on 21 April 2014. Retrieved 20 April 2014.
- ^ Jump up to:a b “Exparel: Pending EC decision”. European Medicines Agency (EMA). 17 September 2020. Retrieved 21 September 2020.Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ “Exparel liposomal EPAR”. European Medicines Agency (EMA). 15 September 2020. Retrieved 11 December 2020.
- ^ “Bupivacaine Effectiveness and Safety in SABER Trial (BESST)”. ClinicalTrials.gov. 20 January 2010. Archived from the original on 2011-12-27. Retrieved 2012-03-01.
External links
- “Bupivacaine”. Drug Information Portal. U.S. National Library of Medicine.
///////////Bupivacaine, AH 250, DUR-843, LAC-43, SKY 0402, SKY-0402, SKY0402, Win 11318, ANAESTHETIC
- CCCCN1CCCCC1C(=O)NC1=C(C)C=CC=C1C
