Verdiperstat
AZD 3241; BHV-3241
CAS No. : 890655-80-8
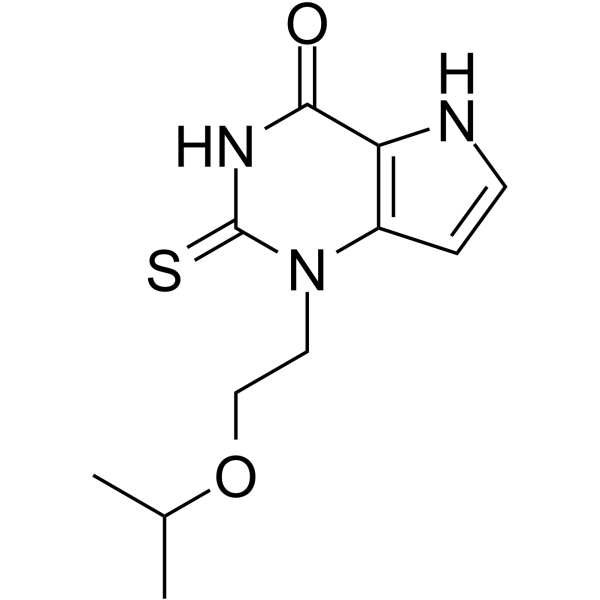
1-(2-propan-2-yloxyethyl)-2-sulfanylidene-5H-pyrrolo[3,2-d]pyrimidin-4-one
4H-Pyrrolo[3,2-d]pyrimidin-4-one, 1,2,3,5-tetrahydro-1-[2-(1-methylethoxy)ethyl]-2-thioxo-
1-(2-isopropoxyethyl)-2-thioxo-1,2,3,5-tetrahydro-pyrrolo[3,2-d] pyrimidin-4-one
l-(2-Isopropoxyethyl)-2-thioxo-l,2,3,5-tetrahydro-pyrrolo[3,2-d]pyrimidin-4-one
- Molecular FormulaC11H15N3O2S
- Average mass253.321 Da
AZD-3241, BHV-3421, UNII-TT3345YXVR, TT3345YXVR, BHV-3241, WHO 10251вердиперстат [Russian] [INN]فيرديبيرستات [Arabic] [INN]维地泊司他 [Chinese] [INN]
- OriginatorAstraZeneca
- DeveloperAstraZeneca; Biohaven Pharmaceuticals
- ClassAntiparkinsonians; Ethers; Organic sulfur compounds; Pyrimidinones; Small molecules
- Mechanism of ActionPeroxidase inhibitors
- Orphan Drug StatusYes – Multiple system atrophy
- Phase IIIMultiple system atrophy
- Phase II/IIIAmyotrophic lateral sclerosis
- DiscontinuedParkinson’s disease
- 23 Jun 20213574186: Added patent info and HE
- 23 Jun 2021Biohaven Pharmaceuticals has patents pending for the composition of matter of verdiperstat, pharmaceutical compositions and various neurological diseases in Europe, Japan and other countries
- 01 Nov 2020Brigham and Women’s Hospital plans a phase I trial for Multiple System Atrophy in USA , (NCT04616456)
EU/3/14/1404: Orphan designation for the treatment of multiple system atrophy
This medicine is now known as verdiperstat.
On 16 December 2014, orphan designation (EU/3/14/1404) was granted by the European Commission to Astra Zeneca AB, Sweden, for 1-(2-isopropoxyethyl)-2-thioxo-1,2,3,5-tetrahydro-pyrrolo[3,2-d] pyrimidin-4-one for the treatment of multiple system atrophy.
The sponsorship was transferred to Richardson Associates Regulatory Affairs Limited, Ireland, in March 2019.
The sponsorship was transferred to Biohaven Pharmaceutical Ireland DAC, Ireland, in September 2021.
Key facts
| Active substance | 1-(2-isopropoxyethyl)-2-thioxo-1,2,3,5-tetrahydro-pyrrolo[3,2-d] pyrimidin-4-one (verdiperstat) |
| Intented use | Treatment of multiple system atrophy |
| Orphan designation status | Positive |
| EU designation number | EU/3/14/1404 |
| Date of designation | 16/12/2014 |
| Sponsor | Biohaven Pharmaceutical Ireland DAC |
VERDIPERSTAT
For Initial Indications in Multiple System Atrophy (MSA) and Amyotrophic Lateral Sclerosis (ALS)
Verdiperstat is a first-in-class, potent, selective, brain-penetrant, irreversible myeloperoxidase (MPO) enzyme inhibitor. Verdiperstat was progressed through Phase 2 clinical trials by AstraZeneca. Seven clinical studies were completed by AstraZeneca, including four Phase 1 studies in healthy subjects, two Phase 2a studies in subjects with Parkinson’s Disease, and one Phase 2b study in subjects with MSA. These Phase 2 clinical studies provide evidence that verdiperstat achieves peripheral target engagement (i.e., reduces MPO specific activity in plasma) and central target engagement in the brain and offer proof of its mechanism of action (i.e., reduce microglial activation and neuroinflamation).
A Phase 3 clinical trial to evaluate the efficacy of verdiperstat in MSA is currently ongoing. A Phase 2/3 trial to evaluate the efficacy of verdiperstat in ALS is currently ongoing as part of the HEALEY ALS Platform Trial.
Verdiperstat has received Fast Track and Orphan Drug designations by the U.S. Food and Drug Administration (FDA) and the European Medicine Agency due to the unmet medical needs in MSA.
Verdiperstat Overview
DESCRIPTIONClick to expendFirst-in-class, brain-penetrant, irreversible inhibitor of MPO
CLINICAL STATUSClick to expendOver 250 healthy volunteers and patients have been treated with verdiperstat in Phase 1 and Phase 2 studies. A Phase 3 study in MSA is currently underway and a Phase 2/3 study in ALS is currently enrolling.
Verdiperstat (AZD3241) is a selective, irreversible and orally active myeloperoxidase (MPO) inhibitor, with an IC50 of 630 nM, and can be used in the research of neurodegenerative brain disorders.

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter a
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp

PATENTWO 2006062465https://patents.google.com/patent/WO2006062465A1/enExample 9 l-(2-Isopropoxyethyl)-2-thioxo-l,2,3,5-tetrahydro-pyrrolo[3,2-d]pyrimidin-4-one (a) 3-[(2-Isopropoxyethyl)ωnino]-lH-pyrwle-2-carboxylic acid ethyl ester Trichlorocyanuric acid (1.84 g, 7.93 mmol) was added to a solution of 2- isopropoxyethanol (0.75 g, 7.21 mmol) in CH2Cl2 (3 mL). The reaction mixture was cooled to 0 °C and TEMPO (0.022 g, 0.14 mmol) was carefully added in small portions. The mixture was stirred at r.t. for 20 minutes then filtered through Celite and washed with CH2Cl2. The filtrate was kept cold, 0 °C, during filtration. The aldehyde solution was added to a stirred mixture of 3-amino-lH-pyrrole-2-carboxylic acid ester (0.83 g, 5.41 mmol) and HOAc (0.62 mL, 10.8 mmol) at 0 °C in methanol (5 mL). The mixture was stirred for 20 minutes, then NaCNBH3 (0.34 g, 5.41 mmol) was added. After stirring at r.t for 2 h, the solution was evaporated onto silica and purified by flash column chromatography (heptane/ethyl acetate gradient; 0 to 100% ethyl acetate) to yield the title compound (0.75 g, 58%) as an oil. 1H NMR (DMSO-d6) δ ppm 10.72 (IH, br s), 6.76-6.74 (IH, m), 5.66-5.65 (IH, m), 5.34(1H, br s), 4.17 (2H, q, J=7.0 Hz), 3.59-3.49 (3H, m), 3.15 (2H, q, J=5.6 Hz), 1.26 (3H, t, J=7.0 Hz), 1.10 (3H, s), 1.08 (3H, s); MS (ESI) m/z 241 (M +1).(b) l-(2-Isopropoxyethyl)-2-thioxo-l,2,3,5-tetrahydro-pyrrolo[3,2-d]pyrimidin-4-one The title compound (0.17 g, 23%) was prepared in accordance with the general method B using 3-[(2-isopropoxyethyl)amino]-lH-pyrrole-2-carboxylic acid ethyl ester (0.7 g, 2.91 mmol) and ethoxycarbonyl isothiocyanate (0.40 mL, 3.50 mmol).1H NMR (DMSO-d6) δ ppm 12.74 (2H, br s), 7.35 (IH, d, J=2.8 Hz), 6.29 (IH, d, J=3.0Hz), 4.49 (2H, t, J=6.3 Hz), 3.72 (2H, t, J=6.3 Hz), 3.60-3.58 (IH, m), 1.02 (3H, s), 1.01 (3H, s);MS (ESI) m/z 254 (M +1).
/////////verdiperstat, вердиперстат , فيرديبيرستات , 维地泊司他 , WHO 10251, AZD-3241, BHV-3421, UNII-TT3345YXVR, TT3345YXVR, BHV-3241, AZD 3241, BHV 3241, BHV 3421
CC(C)OCCN1C2=C(C(=O)NC1=S)NC=C2


















