

Pegvaliase
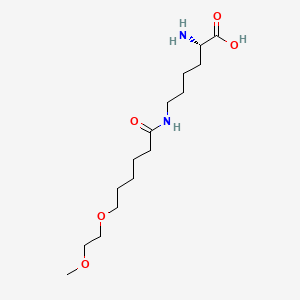
(2S)-2-amino-6-[6-(2-methoxyethoxy)hexanoylamino]hexanoic acid
CAS 1585984-95-7
- Molecular FormulaC15H30N2O5
- Average mass318.409 Da
BMN-165
Palynziq
pegvaliase
pegvaliase-pqpz
L-Lysine, N6-[6-(2-methoxyethoxy)-1-oxohexyl]-
N6-[6-(2-Methoxyethoxy)hexanoyl]-L-lysine
AUSTRALIA APPROVAL 2021
PALYNZIQ
Evaluation commenced: 30 Sep 2020
Registration decision: 6 Jul 2021
Date registered: 14 Jul 2021
Approval time: 166 (175 working days)
pegvaliase
BioMarin Pharmaceutical Australia Pty Ltd
PALYNZIQ (solution for injection, pre-filled syringe) is indicated for the treatment of patients with phenylketonuria (PKU) aged 16 years and older who have inadequate blood phenylalanine control despite prior management with available treatment options.
Pegvaliase, sold under the brand name Palynziq, is a medication for the treatment of the genetic disease phenylketonuria.[2][3] Chemically, it is a pegylated derivative of the enzyme phenylalanine ammonia-lyase that metabolizes phenylalanine to reduce its blood levels.[4]
It was approved by the Food and Drug Administration for use in the United States in 2018.[2] The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[5]
Pegvaliase is a recombinant phenylalanine ammonia lyase (PAL) enzyme derived from Anabaena variabilis that converts phenylalanine to ammonia and trans-cinnamic acid. Both the U.S. Food and Drug Administration and European Medicines Agency approved pegvaliase-pqpz in May 2018 for the treatment of adult patients with phenylketonuria (PKU). Phenylketonuria is a rare autosomal recessive disorder that is characterized by deficiency of the enzyme phenylalanine hydroxylase (PAH) and affects about 1 in 10,000 to 15,000 people in the United States. PAH deficiency and inability to break down an amino acid phenylalanine (Phe) leads to elevated blood phenylalanine concentrations and accumulation of neurotoxic Phe in the brain, causing chronic intellectual, neurodevelopmental and psychiatric disabilities if untreated. Individuals with PKU also need to be under a strictly restricted diet as Phe is present in foods and products with high-intensity sweeteners. The primary goal of lifelong treatment of PKU, as recommended by the American College of Medical Genetics and Genomics (ACMG) guidelines, is to maintain blood Phe concentration in the range of 120 µmol/L to 3690 µmol/L. Pegvaliase-pqpz, or PEGylated pegvaliase, is used as a novel enzyme substitution therapy and is marketed as Palynziq for subcutanoues injection. It is advantageous over currently available management therapies for PKU, such as [DB00360], that are ineffective to many patients due to long-term adherence issues or inadequate Phe-lowering effects. The presence of a PEG moiety in pegvaliase-pqpz allows a reduced immune response and improved pharmacodynamic stability.
References
- ^ Jump up to:a b “Palynziq”. Therapeutic Goods Administration (TGA). 23 July 2021. Retrieved 5 September 2021.
- ^ Jump up to:a b “FDA approves a new treatment for PKU, a rare and serious genetic disease” (Press release). Food and Drug Administration. May 24, 2018.
- ^ Mahan KC, Gandhi MA, Anand S (April 2019). “Pegvaliase: a novel treatment option for adults with phenylketonuria”. Current Medical Research and Opinion. 35 (4): 647–651. doi:10.1080/03007995.2018.1528215. PMID 30247930.
- ^ “Palynziq”. BioMarin Pharmaceutica.
- ^ New Drug Therapy Approvals 2018 (PDF). U.S. Food and Drug Administration (FDA) (Report). January 2019. Retrieved 16 September 2020.
External links
- “Pegvaliase”. Drug Information Portal. U.S. National Library of Medicine.
| Clinical data | |
|---|---|
| Pronunciation | peg val’ i ase |
| Trade names | Palynziq |
| Other names | Pegvaliase-pqpz; PEG-PAL; RAvPAL-PEG |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618057 |
| License data | US DailyMed: Pegvaliase |
| Pregnancy category | AU: D[1] |
| Routes of administration | Subcutaneous |
| ATC code | A16AB19 (WHO) |
| Legal status | |
| Legal status | AU: S4 (Prescription only) [1]US: ℞-onlyEU: Rx-only |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 1585984-95-7 |
| PubChem CID | 86278362 |
| DrugBank | DB12839 |
| ChemSpider | 58172730 |
| UNII | N6UAH27EUV |
| KEGG | D11077 |
| Chemical and physical data | |
| Formula | C15H30N2O5 |
| Molar mass | 318.414 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| showSMILES | |
| showInChI |
////////////pegvaliase, PALYNZIQ, AUSTRALIA 2021, APPROVALS 2021, BioMarin, BMN 165, Palynziq, pegvaliase, pegvaliase-pqpz
COCCOCCCCCC(=O)NCCCCC(C(=O)O)N
