
Bemiparin
- AVE 5026
- Adomiparin
- Ardeparin
- Arteven
- Bemiparin
- CY 216
- CY 222
- Centaxarin
- Certoparin
- Clevarin
- Clivarin
- Clivarine
- Dalteparin
- Deligoparin
- F 202
- FR 860
- Fluxum
- Fragmin A
- Fragmin B
- Fraxiparin
- Gammaparin
- H 5284
- H 9399
- Hapacarin
- Heparin subcutan
- Heparin sulfate
- Heparinic acid
- Heparins
- KB 101
- Leparan
- LipoHep Forte
- Livaracine
- M 118
- M 118REH
- M 402
- M 402 (heparin)
- Mono-embolex
- Multiparin
- Nadroparin
- Nadroparine
- Necuparanib
- Novoheparin
- OP 386
- OP 622
- Octaparin
- Pabyrn
- Parnaparin
- Parvoparin
- Reviparin
- Sandoparin
- Semuloparin
- Subeparin
- Sublingula
- Tafoxiparin
- Tinzaparin
- Triofiban
- Vetren
- Vitrum AB
- α-Heparin
cas 91449-79-5

Bemiparin (trade names Ivor and Zibor, among others) is an antithrombotic and belongs to the group of low molecular weight heparins (LMWH).[1]
Bemiparin is an ultra-low molecular weight heparin (ultra-LMWH) used to prevent thromboembolism following surgery and extracorporeal clotting during dialysis.
Rovi and Archimedes (a wholly owned subsidiary of ProStrakan), have developed and launched bemiparin, a Factor Xa inhibitor for the injectable treatment and prevention of thrombosis.
low or very low molecular weight heparins (eg bemiparin sodium) with a high anti-factor Xa activity for the treatment of deep vein thrombosis.
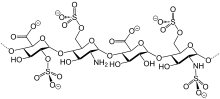
Bemiparin is an antithrombotic and belongs to the group of drugs known as the low molecular weight heparins (LMWH). Like semuloparin, bemiparin is classified as an ultra-LMH because of its low mean molecular mass of 3600 daltons, which is a unique property of this class 1. These heparins have lower anti-thrombin activity than the traditional low molecular weight heparins and act mainly on factor-Xa, reducing the risk of bleeding due to selectivity for this specific clotting factor. Interestingly, current research is underway for the potential benefit of bemiparin in the treatment of tumors and diabetic foot ulcers 12,1.
Laboratorios Farmaceuticos Rovi has developed and launched Enoxaparina Rovi, a biosimilar version of enoxaparin sodium, an injectable low-molecular-weight fraction of heparin, for the prophylaxis of venous thromboembolism.
PATENT
WO2018015463
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2018015463
claiming a method for analyzing glycosaminoglycans, heparins and their derivatives in a compound comprising a monosaccharide residues present in heparin (eg bemiparin sodium) chains by identification and relative quantification of its characteristic signals by1H NMR one-dimensional nuclear magnetic resonance and/or 1H-13C HSQC two-dimensional nuclear magnetic resonance, using dimethylmalonic acid as internal reference
PATENT
CN-110092848
https://patents.google.com/patent/CN110092848A/enEmbodiment 1Experimental raw used and instrument are as follows in embodiment 1:Refined heparin sodium (ZH160712 quality of lot meets CP2015), benzethonium chloride, purified water, 40% (W/V) trimethoxy Base methanolic ammonium hydroxide, methylene chloride, methanol, 10% (W/V) sodium acetate methanol solution, 30% hydrogen peroxide, medicinal second Alcohol, sodium chloride, glass reaction pot (5000ml) three-necked flask 500ml, digital display heat-collecting magnetic stirring device, beaker, freeze dryer (on Hai Dongfulong) etc..A kind of preparation method of Bemiparin sodium of the present invention, the following steps are included:1. at salt1.1 weigh, dissolution, react1.1.1 the refined heparin sodium for weighing 10g is poured into tank, and the purified water of 100ml is added into reactor tank, is stirred to molten Solution is complete.1.1.2 25g benzethonium chloride is added in beaker, 125ml purified water stirring and dissolving is added.1.1.3 benzethonium chloride solution is added slowly with stirring in the heparin sodium aqua in reactor tank, time for adding 4.5h controls 35 DEG C of feed liquid temperature, continues stirring 2 hours, stops stirring and stands 2 hours, then as far as possible by supernatant liquid Removing.1.2 washings, centrifugation, drying:1.2.1 300ml purified water is added into residue precipitating suspended matter to wash in three times, then starts to wash for the first time, 20 DEG C of feed liquid temperature of control is stirred 1 hour, is stopped stirring and is stood 2 hours, repeats the above operation twice.1.2.2 supernatant liquid is removed, filters and be washed with water under stirring, record slurry amount, collect sediment.1.2.3 final gained sediment is uniformly divided in stainless steel disc, is transferred in heated-air circulation oven, adjust temperature 40 DEG C of degree, dry 6h crushes solid with Universalpulverizer after then 60 DEG C of dry range estimations are not glued to solid, smashed solid Body continues to be transferred in heated-air circulation oven, until loss on drying≤2.0%.Rewinding obtains heparin-benzyl rope ammonium salt about 32g, does Dry weightless 1.5%.2. degradation2.1 weighingBy above-mentioned 30g heparin-benzyl rope ammonium salt in 500ml three-necked flask, the methylene chloride of 150ml is added into reactor tank It is added in three-necked flask.2.2 dissolutions: three-necked flask is put into digital display heat-collecting magnetic stirring device, is heated to 33 DEG C and is stirred to having dissolved Entirely.2.3 degradations: being added 40% (W/V) trimethoxy methanolic ammonium hydroxide of 20.4ml in Xiang Shangshu solution, puts down Respectively 4 additions, it is for 24 hours that interval time is added every time.It after the 4th is added, then reacts for 24 hours, amounts to reaction 96h, during reaction Maintain 34 DEG C of temperature.2.4 terminate reaction: above-mentioned reaction solution being cooled to 20 DEG C, 180ml10% (W/V) sodium acetate methanol is added thereto Solution stirs 30min, filters to obtain its precipitating.2.5 washings: washing above-mentioned sediment with 300ml methanol solution, dry bemiparin crude product about 9g.3. purification3.1 will be above-mentioned dry that 9g bemiparin crude product pours into tank, and the purified water of 90ml, stirring are added into reactor tank It is complete to dissolution.3.2 adjust material liquid pH 9.5 with 20% sodium hydroxide solution.0.54ml hydrogen peroxide is added to be stirred to react at 20 DEG C 7.5 hours, through 0.22 μm of micro porous filtration.3.3 1.8g sodium chloride is added into feed liquid, then uses 4mol/L hydrochloric acid flavouring liquid pH to 6.5, is added into feed liquid 450ml medicinal alcohol stops stirring after stirring 30 minutes, places 4 hours.3.4 take supernatant away, and 90ml purified water is added, and stirring adjusts PH6.5 to dissolving completely, through 0.22 μm of micro porous filtration, Sabot freeze-drying.After 3.5 freeze-drying 36h, collection material weighing 7g.Three, the primary quality measure statistics of gained bemiparin
| Serial number | Project | Control standard | Testing result |
| 1 | Weight average molecular weight | 3000~4200 | 3650 |
| 2 | Molecular weight is greater than 6000 constituent content | < 15% | 12.9% |
| 3 | Constituent content of the molecular weight less than 2000 | < 35% | 36.7% |
| 4 | Molecular weight is between 2000~6000 constituent contents | 50%~75% | 50.4% |
| 5 | Anti-Xa activity | 80~120IU/mg | 116IU/mg |
| 6 | Anti- IIa activity | 5~20IU/mg | 14.6IU/mg |
| 7 | The anti-anti- IIa of Xa/ | ≥7 | 7.95 |
Embodiment 2Experimental raw used and instrument are as follows in embodiment 1:Refined heparin sodium (ZH180912 quality of lot meets CP2015), benzethonium chloride, purified water, 40% (W/V) trimethoxy Base methanolic ammonium hydroxide, methylene chloride, methanol, 10% (W/V) sodium acetate methanol solution, 30% hydrogen peroxide, medicinal second Alcohol, sodium chloride, glass reaction pot (10000ml, 30000L), three-necked flask 500ml, digital display heat-collecting magnetic stirring device, beaker, Freeze dryer (Shanghai Dong Fulong) etc..A kind of preparation method of Bemiparin sodium of the present invention, the following steps are included: 1. one-tenth salt1.1 weigh, dissolution, react1.1.1 the refined heparin sodium for weighing 500g is poured into tank, the purified water of 5000ml is added into reactor tank, stirring is extremely Dissolution is complete.1.1.2 1250g benzethonium chloride is added in beaker, 6300ml purified water stirring and dissolving is added.1.1.3 benzethonium chloride solution is added slowly with stirring in the heparin sodium aqua in reactor tank, time for adding 5h controls 35 DEG C of feed liquid temperature, continues stirring 2 hours, stops stirring and stands 2 hours, then as far as possible by supernatant liquid It removes.1.2 washings, centrifugation, drying:1.2.1 5000ml purified water is added into residue precipitating suspended matter to wash in three times, then starts to wash for the first time, 30 DEG C of feed liquid temperature of control is stirred 1 hour, is stopped stirring and is stood 2 hours, repeats the above operation twice.1.2.2 supernatant liquid is removed, filters and be washed with water under stirring, record slurry amount, collect sediment.1.2.3 final gained sediment is uniformly divided in stainless steel disc, is transferred in heated-air circulation oven, adjust temperature 45 DEG C of degree, dry 6h crushes solid with Universalpulverizer after then 70 DEG C of dry range estimations are not glued to solid, smashed solid Body continues to be transferred in heated-air circulation oven, until loss on drying≤2.0%.Rewinding obtains heparin-benzyl rope ammonium salt about 1505g, Loss on drying 1.0%.2. degradation2.1 weighingBy above-mentioned 1500g heparin-benzyl rope ammonium salt in 30L glass reaction kettle, the methylene chloride of 7500ml is added thereto.2.2 dissolutions: leading to hot water for its interlayer, is heated to 33~36 DEG C and stirs complete to dissolving.2.3 degradations: being added 40% (W/V) trimethoxy methanolic ammonium hydroxide of 1020ml in Xiang Shangshu solution, puts down Respectively 4 additions, it is for 24 hours that interval time is added every time.It after the 4th is added, then reacts for 24 hours, amounts to reaction 96h, during reaction Maintain 35 DEG C of temperature.2.4 terminate reaction: above-mentioned reaction solution being cooled to 20 DEG C, 9000ml10% (W/V) sodium acetate first is added thereto Alcoholic solution stirs 30min, filters to obtain its precipitating.2.5 washings: washing above-mentioned sediment with 15000ml methanol solution, dry bemiparin crude product about 400g.3. purification3.1 will be above-mentioned dry that 400g bemiparin crude product pours into tank, and the purified water of 4000ml is added into reactor tank, Stirring is complete to dissolving.3.2 adjust material liquid pH 9.5 with 20% sodium hydroxide solution.24ml hydrogen peroxide is added, and at 30 DEG C to be stirred to react 7 small When, through 0.22 μm of micro porous filtration.3.3 8g sodium chloride is added into feed liquid, then uses 4mol/L hydrochloric acid flavouring liquid pH to 6.5, is added into feed liquid 20000ml medicinal alcohol stops stirring after stirring 30 minutes, places 4 hours.3.4 take supernatant away, and 4000ml purified water is added, and stirring adjusts PH6.5, through 0.22 μm of micropore mistake to dissolving completely Filter, sabot freeze-drying.After 3.5 freeze-drying 36h, collection material weighing 350g.Three, the primary quality measure statistics of gained bemiparin

PATENT
WO-2021152192
https://patentscope.wipo.int/search/en/detail.jsf;jsessionid=9D96E01E1CE8B8107A83A95B4B344DD3.wapp2nC?docId=WO2021152192&tab=PCTDESCRIPTION
Use of a composition comprising low or very low molecular weight heparins (eg bemiparin sodium) with a high anti-factor Xa activity for the treatment of deep vein thrombosis.
Heparin belongs to the glycosaminoglycan family and is a polysaccharide of animal origin, which is extracted from the intestine or lungs of mammals (cow, lamb, pig) and is used in human therapies for the prevention and treatment of thromboembolic diseases . It is well known that the use of heparin is accompanied by very annoying bleeding effects and its daily administration, three subcutaneous or intravenous injections, constitutes a very considerable inconvenience.
During the course of the last few years, different chemical methods have been used to depolymerize heparin, such as:
– treatment with sodium nitrite in an acid medium,
– alkaline treatment of asters,
– use of free radicals generated in the presence of hydrogen peroxide,
– treatment of a quaternary ammonium salt of heparin in a non-aqueous medium with a strong base according to a beta elimination mechanism.
These methods make it possible to obtain, with variable yields, mixtures of heparin fragments in which the average molecular weight and anticoagulant activity vary according to the procedure and operating conditions. Low molecular weight heparins (LMWH) described in the state of the art or commercialized are obtained according to different depolymerization procedures. Their average molecular weights (Mw) are in the range of 3,600 and 7,500 Daltons.
It is now recognized that the antithrombotic activity of LMWH is mainly due to its ability to activate antithrombin III, a plasma protein and potent inhibitor of activated factor X and thrombin. In this way, it is possible to measure the antithrombotic activity of heparin by means of specific tests to determine the inhibition of these factors.
Research carried out by different authors shows that heparin fragments or oligosaccharides, with short chains of average molecular weight <4,800 Daltons, have a selective action on activated factor X and not on thrombin, in determinations using methods of the Pharmacopoeia. .
It has been found that if very low molecular weight fragments are required that have strong anti-factor Xa activity, it is preferable to use a selective depolymerization technique in non-aqueous medium, as described in US patent 9,981,955, which respects the antithrombin III binding site.
The document EP 1070503 A1 describes the controlled depolymerization of heparin using a process in a non-aqueous medium that makes it possible to obtain a family of LMWH that are obtained enriched in low molecular weight oligosaccharides that have a high anti-factor Xa activity and a low anti-factor lia activity, and which can be represented by the general formula:
in which:
n can vary between 1 and 12,
Ri = H or S0 3 Na,
R 2 = SOsNao COCH 3 ,
Said very low molecular weight heparin is obtained by selective depolymerization of heparin in a non-aqueous medium according to a beta elimination procedure.
Medical uses
Bemiparin is used for the prevention of thromboembolism after surgery, and to prevent blood clotting in the extracorporeal circuit in haemodialysis.[2]
Contraindications
The medication is contraindicated in patients with a history of heparin-induced thrombocytopenia with or without disseminated intravascular coagulation; acute bleeding or risk of bleeding; injury or surgery of the central nervous system, eyes or ears; severe liver or pancreas impairment; and acute or subacute bacterial endocarditis.[2]
Interactions
No interaction studies have been conducted. Drugs that are expected to increase the risk of bleeding in combination with bemiparin include other anticoagulants, aspirin and other NSAIDs, antiplatelet drugs, and corticosteroids.[2]
Chemistry
Like semuloparin, bemiparin is classified as an ultra-LMWH because of its low molecular mass of 3600 g/mol on average.[3] (Enoxaparin has 4500 g/mol.) These heparins have lower anti-thrombin activity than classical LMWHs and act mainly on factor Xa, reducing the risk of bleeding.[4]
References
- ^ Chapman TM, Goa KL (2003). “Bemiparin: a review of its use in the prevention of venous thromboembolism and treatment of deep vein thrombosis”. Drugs. 63 (21): 2357–77. doi:10.2165/00003495-200363210-00009. PMID 14524738.
- ^ Jump up to:a b c Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag. 2018. Ivor 2500 IE Anti-Xa/0,2 ml Injektionslösung in Fertigspritzen.
- ^ Planès A (September 2003). “Review of bemiparin sodium–a new second-generation low molecular weight heparin and its applications in venous thromboembolism”. Expert Opinion on Pharmacotherapy. 4 (9): 1551–61. doi:10.1517/14656566.4.9.1551. PMID 12943485. S2CID 13566575.
- ^ Jeske WP, Hoppensteadt D, Gray A, Walenga JM, Cunanan J, Myers L, Fareed J, Bayol A, Rigal H, Viskov C (October 2011). “A common standard is inappropriate for determining the potency of ultra low molecular weight heparins such as semuloparin and bemiparin”. Thrombosis Research. 128 (4): 361–7. doi:10.1016/j.thromres.2011.03.001. PMID 21458847.
External links
- bemiparin at the US National Library of Medicine Medical Subject Headings (MeSH)
| Clinical data | |
|---|---|
| Trade names | Badyket, Ivor, Hibor, Zibor, others |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Subcutaneous injection (except for haemodialysis) |
| ATC code | B01AB12 (WHO) |
| Pharmacokinetic data | |
| Bioavailability | 96% (estimated) |
| Elimination half-life | 5–6 hours |
| Identifiers | |
| CAS Number | 91449-79-5 |
| DrugBank | DB09258 |
| ChemSpider | none |
| Chemical and physical data | |
| Molar mass | 3600 g/mol (average) |
| (what is this?) (verify) |
- Chapman TM, Goa KL: Bemiparin: a review of its use in the prevention of venous thromboembolism and treatment of deep vein thrombosis. Drugs. 2003;63(21):2357-77. [Article]
- Planes A: Review of bemiparin sodium–a new second-generation low molecular weight heparin and its applications in venous thromboembolism. Expert Opin Pharmacother. 2003 Sep;4(9):1551-61. [Article]
- Jeske WP, Hoppensteadt D, Gray A, Walenga JM, Cunanan J, Myers L, Fareed J, Bayol A, Rigal H, Viskov C: A common standard is inappropriate for determining the potency of ultra low molecular weight heparins such as semuloparin and bemiparin. Thromb Res. 2011 Oct;128(4):361-7. doi: 10.1016/j.thromres.2011.03.001. Epub 2011 Apr 2. [Article]
- Sanchez-Ferrer CF: Bemiparin: pharmacological profile. Drugs. 2010 Dec 14;70 Suppl 2:19-23. doi: 10.2165/1158581-S0-000000000-00000. [Article]
- Hoffman M, Monroe DM: Coagulation 2006: a modern view of hemostasis. Hematol Oncol Clin North Am. 2007 Feb;21(1):1-11. doi: 10.1016/j.hoc.2006.11.004. [Article]
- Antonijoan RM, Rico S, Martinez-Gonzalez J, Borrell M, Valcarcel D, Fontcuberta J, Barbanoj MJ: Comparative pharmacodynamic time-course of bemiparin and enoxaparin in healthy volunteers. Int J Clin Pharmacol Ther. 2009 Dec;47(12):726-32. [Article]
- Irish Medicines Board: Bemiparin [Link]
- Hibor-Bemiparin Sodium [Link]
- Zibor 2,500 IU Solution for Injection [Link]
- Injectable drugs guide [Link]
- Thrombosis Advisors- Factor Xa inhibitor [Link]
- Anti-tumor effects of bemiparin in HepG2 and MIA PaCa-2 cells [Link]
- Bemiparin, an effective and safe low molecular weight heparin: a review [Link]
- Bemiparin sodium [Link]
Patent
Publication numberPriority datePublication dateAssigneeTitleUS4981955A *1988-06-281991-01-01Lopez Lorenzo LDepolymerization method of heparinEP0293539B1 *1987-01-051994-06-08Laboratorios Farmaceuticos Rovi, S.A.Process for the depolymerization of heparin for obtaining heparin with a low molecular weight and having an antithrombotic activityCN1379781A *1999-10-222002-11-13阿文蒂斯药物股份有限公司Novel oligosaccharides, preparation method and pharmaceutical composition containing sameCN102399306A *2010-09-092012-04-04上海喜恩医药科技发展有限公司Preparation method of heparin-derived polysaccharide mixtureCN105693886A *2016-04-192016-06-22常州市蓝勖化工有限公司Preparation method of heparin sodiumCN106467577A *2015-08-212017-03-01苏州融析生物科技有限公司A kind of pulmonis Bovis seu Bubali Enoxaparin Sodium and preparation method and applicationCN106977627A *2017-05-162017-07-25苏州二叶制药有限公司A kind of Enoxaparin production method of sodiumCN109575156A *2018-11-052019-04-05上海宝维医药技术有限公司A kind of purification process of low molecular weight heparinFamily To Family Citations
////////////Bemiparin sodium, Bemiparin