
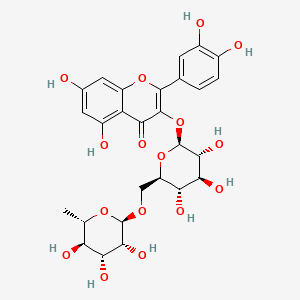

Rutoside
RUTIN
- Molecular FormulaC27H30O16
- Average mass610.518
- рутозид [Russian] [INN]ルチン [Japanese]روتوسيد [Arabic] [INN]芦丁 [Chinese] [INN]
CAS 153-18-4
- C.I. 75730
- NSC-9220
2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxychromen-4-one


Rutin trihydrate | CAS 250249-75-3 RutinCAS Registry Number: 153-18-4
CAS Name: 3-[[6-O-(6-Deoxy-a-L-mannopyranosyl)-b-D-glucopyranosyl]oxy]-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-1-benzopyran-4-one
Additional Names: rutoside; quercetin-3-rutinoside; 3,3¢,4¢,5,7-pentahydroxyflavone-3-rutinoside; melin; phytomelin; eldrin; ilixathin; sophorin; globularicitrin; paliuroside; osyritrin; osyritin; myrticolorin; violaquercitrin
Trademarks: Birutan (Merck KGaA)
Molecular Formula: C27H30O16Molecular Weight: 610.52Percent Composition: C 53.12%, H 4.95%, O 41.93%
Literature References: Identity with ilixanthin: Schindler, Herb, Arch. Pharm.288, 372 (1955). Found in many plants, especially the buckwheat plant (Fagopyrum esculentum Moench., Polygonaceae) which contains about 3% (dry basis): Couch et al.,Science103, 197 (1946). From tobacco (Nicotiana tabacum L., Solanaceae) Couch, Krewson, U.S. Dept. Agr., Eastern Regional Res. Lab.AIC-52 (1944).
In forsythia [Forsythia suspensa (Thunb.) Vahl. var. fortunei (Lindl.) Rehd., Oleaceae], in hydrangea (Hydrangea paniculata Sieb., Saxifragaceae), in pansies (Viola sp., Violaceae).
General extraction procedure: BeilsteinXXXI, 376. From leaves of Eucalyptus macroryncha F. v. Muell., Myrtaceae: Attree, Perkin, J. Chem. Soc.1927, 234.
Industrial production from Eucalyptus spp.: Humphreys, Econ. Bot.18, 195 (1964).
Structure: Zemplén, Gerecs, Ber.68B, 1318 (1935).
Synthesis: Shakhova et al.,Zh. Obshch. Khim.32, 390 (1962), C.A.58, 1426e (1963). Rutin is hydrolyzed by rhamnodiastase from the seed of Rhamnus utilis Decne, Rhamnaceae (Chinese buckthorn); emulsin is not effective: Bridel, Charaux, Compt. Rend.181, 925 (1925). Toxicity data: Harrison et al.,J. Am. Pharm. Assoc.39, 557 (1950). Book: J. Q. Griffith, Jr., Rutin and Related Flavonoids (Mack, Easton, Pa., 1955).
Comprehensive description: T. I. Khalifa et al.,Anal. Profiles Drug Subs.12, 623-681 (1983).UV






Properties: Pale yellow needles from water, gradual darkening on exposure to light. The crystals contain 3 H2O and become anhydr after 12 hrs at 110° and 10 mm Hg. Anhydr rutin browns at 125°, becomes plastic at 195-197°, and dec 214-215° (with effervescence). [a]D23 +13.82° (ethanol); [a]D23 -39.43° (pyridine). Anhydr rutin is hygroscopic. One gram dissolves in about 8 liters water, about 200 ml boiling water, 7 ml boiling methanol. Sol in pyridine, formamide and alkaline solns; slightly sol in alcohol, acetone, ethyl acetate. Practically insol in chloroform, carbon bisulfide, ether, benzene, petr solvents. Dil solns give green color with ferric chloride. Rutin is colored brown by tobacco enzyme under experimental conditions: Neuberg, Kobel, Naturwissenschaften23, 800 (1935). LD50 i.v. in mice: 950 mg/kg (propylene glycol soln) (Harrison).
Optical Rotation: [a]D23 +13.82° (ethanol); [a]D23 -39.43° (pyridine)
Toxicity data: LD50 i.v. in mice: 950 mg/kg (propylene glycol soln) (Harrison)Therap-Cat: Capillary protectant.Keywords: Vasoprotectant.
C13




MASS


Rutin, also called rutoside, quercetin-3-O-rutinoside and sophorin, is the glycoside combining the flavonol quercetin and the disaccharide rutinose (α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranose). It is a citrus flavonoid found in a wide variety of plants including citrus.
Rutin, also called rutoside, is the glycoside flavonoid found in a certain fruits and vegetables. Most rutine-rich foods are capers, olives, buckwheat (whole grain flour), asparagus, raspberry.In a clinical trial, rutin was found to aid control of intraocular pressure in patients with primary open angle glaucoma. As a component of dietary supplement Phlogenzym, rutin is used for treatment of osteoarthritis. Rutin is also used for treatment of post-surgical swelling of the arm after breast cancer surgery. Traditionally, rutin is used to prevent mucositis due to cancer treatment, to treat blood vessel disease such as varicose veins, bleeding, hemorrhoids.
Occurrences
Rutin is one of the phenolic compounds found in the invasive plant species Carpobrotus edulis and contributes to the antibacterial[3] properties of the plant.
Its name comes from the name of Ruta graveolens, a plant that also contains rutin.
Various citrus fruit peels contain 32 to 49 mg/g of flavonoids expressed as rutin equivalents.[4]
Citrus leaves contain rutin at concentrations of 11 and 7 g/kg in orange and lime trees respectively.[5]
Metabolism
The enzyme quercitrinase can be found in Aspergillus flavus.[6] It is an enzyme in the rutin catabolic pathway.[7]
In food
Rutin is a citrus flavonoid glycoside found in many plants including buckwheat,[8] the leaves and petioles of Rheum species, and asparagus. Tartary buckwheat seeds have been found to contain more rutin (about 0.8–1.7% dry weight) than common buckwheat seeds (0.01% dry weight).[8] Rutin is one of the primary flavonols found in ‘clingstone’ peaches.[9] It is also found in green tea infusions.[10]
Approximate rutin content per 100g of selected foods, in milligrams per 100 milliliters:[11]
| Numeric | Alphabetic |
|---|---|
| 332 | Capers, spice |
| 45 | Olive [Black], raw |
| 36 | Buckwheat, whole grain flour |
| 23 | Asparagus, raw |
| 19 | Black raspberry, raw |
| 11 | Red raspberry, raw |
| 9 | Buckwheat, groats, thermally treated |
| 6 | Buckwheat, refined flour |
| 6 | Greencurrant |
| 6 | Plum, fresh |
| 5 | Blackcurrant, raw |
| 4 | Blackberry, raw |
| 3 | Tomato (Cherry), whole, raw |
| 2 | Prune |
| 2 | Fenugreek |
| 2 | Marjoram, dried |
| 2 | Tea (Black), infusion |
| 1 | Grape, raisin |
| 1 | Zucchini, raw |
| 1 | Apricot, raw |
| 1 | Tea (Green), infusion |
| 0 | Apple |
| 0 | Redcurrant |
| 0 | Grape (green) |
| 0 | Tomato, whole, raw |
Research
Rutin (rutoside or rutinoside)[12] and other dietary flavonols are under preliminary clinical research for their potential biological effects, such as in reducing post-thrombotic syndrome, venous insufficiency, or endothelial dysfunction, but there was no high-quality evidence for their safe and effective uses as of 2018.[12][13][14][needs update] As a flavonol among similar flavonoids, rutin has low bioavailability due to poor absorption, high metabolism, and rapid excretion that collectively make its potential for use as a therapeutic agent limited.[12]
Biosynthesis
The biosynthesis pathway of rutin in mulberry (Morus alba L.) leaves begins with phenylalanine, which produces cinnamic acid under the action of phenylalanine ammonia lyase (PAL). Cinnamic acid is catalyzed by cinnamic acid-4-hydroxylase (C4H) and 4-coumarate-CoA ligase (4CL) to form p–coumaroyl-CoA. Subsequently, chalcone synthase (CHS) catalyzes the condensation of p-coumaroyl-CoA and three molecules of malonyl-CoA to produce naringenin chalcone, which is eventually converted into naringenin flavanone with the participation of chalcone isomerase (CHI). With the action of flavanone 3-hydroxylas (F3H), dihydrokaempferol (DHK) is generated. DHK can be further hydroxylated by flavonoid 3´-hydroxylase (F3’H) to produce dihydroquercetin (DHQ), which is then catalyzed by flavonol synthase (FLS) to form quercetin. After quercetin is catalyzed by UDP-glucose flavonoid 3-O-glucosyltransferase (UFGT) to form isoquercitrin, finally, the formation of rutin from isoquercitrin is catalyzed by flavonoid 3-O-glucoside L-rhamnosyltransferase.[15]

SYN
https://www.sciencedirect.com/science/article/abs/pii/S100184171300017X
The compound 2 was synthesized for the first time by highly selective esterification reaction and fully characterized. The by-products of the reaction were complex, which brought out many considerable difficulties in separation and purification of the target product. Our work was the first in using the improved pyrogallol autoxidation method to test the antioxidant activities of these two flavonoids compounds in vitro and discovered that the compound 2 was much more effective as a free radical scavenger than the compound 1.

SYN
Synthesis of Rutin
The synthesis of rutin can be achieved according to the following three schemes. These schemes differ in the synthesis of ouercetin (the aglycone moiety of rutin).
Scheme 1: Kostanecki — et al. 1904 (33 ). Based upon the Claisen reaction between 2-hydroxy4, 6-dimethoxyacetophenone [l] and 3, 4-dimethoxybenzaldehyde [2] to give the intermediate [3] which upon treatment with HC1, cyclization occurs to give 5, 7, 3 , which upon treatment with F2SO4 enolisation occurs to give 5, 7, 1’3 ,’4 -tetramethoxyflavonol [6]. tion with HI affords quercetin [7].
Scheme 2: Robinson et al. 1926 (34 ). , ‘4 -tetramethoxyflavonone [4]. Oximination affords [5] Demethyla- — Condensation ofw-methoxypholoroacetophenone [I] with veratric acid anhydride [2] in the presence of the potassium salt of veratric acid to give the diarylester [3]. On hydrolysis with alcoholic KOH affords 5, 7-dihydroxy-3, /3 , ‘4 -trimethoxyf lavone [ 41 , which on demethylation with HI gives quercetin [5].
Scheme 3: Shakhova et al. 1962 (35), complete synthesis of rutin. W-methoxyphloroacetophenone [2] was condensed with 0-benzylvanillinic acid, anhydride [ 13 in triethylamine to give 5 , 7-dihydroxy-4 -benzyloxy-3, /3 -dimethoxyf lavone [3]. On treatment with AcOH-HC1 mixture gave 5, 7, ‘4 -trihydroxy-3,’3 -dimethoxyflavone [4]. Demethylation of the latter with HI yielded (about 802) quercetin [5]. Ouercetin potassium salt [6] was produced upon treating [5] with AcOK in ethanol. Levoglucosan [7] was acetylated with Ac20 in the presence of AcONa to give 2, 3, 4-triacetyllevoglucosan [8] which with TIC14 gave 1-chloro-2, 3, 4-triacetyl Dglucose [9]. L-rhamnose tetraacetate [lo] treated with TiBr4 in CHC13 gave 1-bromo-2, 3, I-triacetyl-L-rhamnose [ll]. [lo] + [11] heated with Hg (OAC)~ in C6H6 gave (53x) CC – acetochloro-f3-l-L-rhamnosido-6-D-glucose [12]. [12] was treated with AgOAc and acetylated with Ac20 to prodilce (68.703 B-heptaacet yl-f3-1-L-rhamnos ido-6-D-glucose [13]. This with 33% HBr in AcOH gave (61%) d – acetobromo-~-l-L-rhamnosido-6-D-glucose [14]. [14] and quercetin potassium salt [6] were dissolved in NH40H which was evaporated and treated with methanol andpurified over a chromatographic column packed with polycaprolactum resin to give rutin [151.



References
- ^ Merck Index, 12th Edition, 8456
- ^ Krewson CF, Naghski J (Nov 1952). “Some physical properties of rutin”. Journal of the American Pharmaceutical Association. 41 (11): 582–7. doi:10.1002/jps.3030411106. PMID 12999623.
- ^ van der Watt E, Pretorius JC (2001). “Purification and identification of active antibacterial components in Carpobrotusedulis L.”. Journal of Ethnopharmacology. 76 (1): 87–91. doi:10.1016/S0378-8741(01)00197-0. PMID 11378287.
- ^ [1] p. 280 Table 1
- ^ [2] p.8 fig. 7
- ^ quercitrinase on www.brenda-enzymes.org
- ^ Tranchimand S, Brouant P, Iacazio G (Nov 2010). “The rutin catabolic pathway with special emphasis on quercetinase”. Biodegradation. 21 (6): 833–59. doi:10.1007/s10532-010-9359-7. PMID 20419500. S2CID 30101803.
- ^ Jump up to:a b Kreft S, Knapp M, Kreft I (Nov 1999). “Extraction of rutin from buckwheat (Fagopyrum esculentumMoench) seeds and determination by capillary electrophoresis”. Journal of Agricultural and Food Chemistry. 47 (11): 4649–52. doi:10.1021/jf990186p. PMID 10552865.
- ^ Chang S, Tan C, Frankel EN, Barrett DM (Feb 2000). “Low-density lipoprotein antioxidant activity of phenolic compounds and polyphenol oxidase activity in selected clingstone peach cultivars”. Journal of Agricultural and Food Chemistry. 48 (2): 147–51. doi:10.1021/jf9904564. PMID 10691607.
- ^ Malagutti AR, Zuin V, Cavalheiro ÉT, Henrique Mazo L (2006). “Determination of Rutin in Green Tea Infusions Using Square-Wave Voltammetry with a Rigid Carbon-Polyurethane Composite Electrode”. Electroanalysis. 18 (10): 1028–1034. doi:10.1002/elan.200603496.
- ^ “foods in which the polyphenol Quercetin 3-O-rutinoside is found”. Phenol-Explorer v 3.6. June 2015.
- ^ Jump up to:a b c “Flavonoids”. Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, Oregon. November 2015. Retrieved 25 February 2018.
- ^ Morling, J. R; Yeoh, S. E; Kolbach, D. N (November 2018). “Rutosides for treatment of post-thrombotic syndrome”. Cochrane Database of Systematic Reviews. 11 (11): CD005625. doi:10.1002/14651858.CD005625.pub4. PMC 6517027. PMID 30406640.
- ^ Martinez-Zapata, M. J; Vernooij, R. W; Uriona Tuma, S. M; Stein, A. T; Moreno, R. M; Vargas, E; Capellà, D; Bonfill Cosp, X (2016). “Phlebotonics for venous insufficiency”. Cochrane Database of Systematic Reviews. 4: CD003229. doi:10.1002/14651858.CD003229.pub3. PMC 7173720. PMID 27048768.
- ^ Yu X, Liu J, Wan J, Zhao L, Liu Y, Wei Y, Ouyang Z. Cloning, prokaryotic expression, and enzyme activity of a UDP-glucose flavonoid 3-o-glycosyltransferase from mulberry (Morus alba L.) leaves. Phcog Mag 2020;16:441-7
| Names | |
|---|---|
| IUPAC name3′,4′,5,7-Tetrahydroxy-3-[α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranosyloxy]flavone | |
| Preferred IUPAC name(42S,43R,44S,45S,46R,72R,73R,74R,75R,76S)-13,14,25,27,43,44,45,73,74,75-Decahydroxy-76-methyl-24H-3,6-dioxa-2(2,3)-[1]benzopyrana-4(2,6),7(2)-bis(oxana)-1(1)-benzenaheptaphane-24-one | |
| Other namesRutoside (INN) Phytomelin Sophorin Birutan Eldrin Birutan Forte Rutin trihydrate Globularicitrin Violaquercitrin Quercetin rutinoside | |
| Identifiers | |
| CAS Number | 153-18-4 |
| 3D model (JSmol) | Interactive image |
| ChemSpider | 4444362 |
| DrugBank | DB01698 |
| ECHA InfoCard | 100.005.287 |
| KEGG | C05625 |
| PubChem CID | 5280805 |
| RTECS number | VM2975000 |
| UNII | 5G06TVY3R7 |
| CompTox Dashboard (EPA) | DTXSID3022326 |
| showInChI | |
| showSMILES | |
| Properties | |
| Chemical formula | C27H30O16 |
| Molar mass | 610.521 g·mol−1 |
| Appearance | Solid |
| Melting point | 242 °C (468 °F; 515 K) |
| Solubility in water | 12.5 mg/100 mL[1] 13 mg/100mL[2] |
| Pharmacology | |
| ATC code | C05CA01 (WHO) |
| Hazards | |
| NFPA 704 (fire diamond) |  200 200 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
 verify (what is ?) verify (what is ?) | |
| Infobox references |
/////////Rutoside, RUTIN, рутозид , ルチン , روتوسيد , 芦丁 , C.I. 75730, NSC 9220,
CC1C(C(C(C(O1)OCC2C(C(C(C(O2)OC3=C(OC4=CC(=CC(=C4C3=O)O)O)C5=CC(=C(C=C5)O)O)O)O)O)O)O)O






