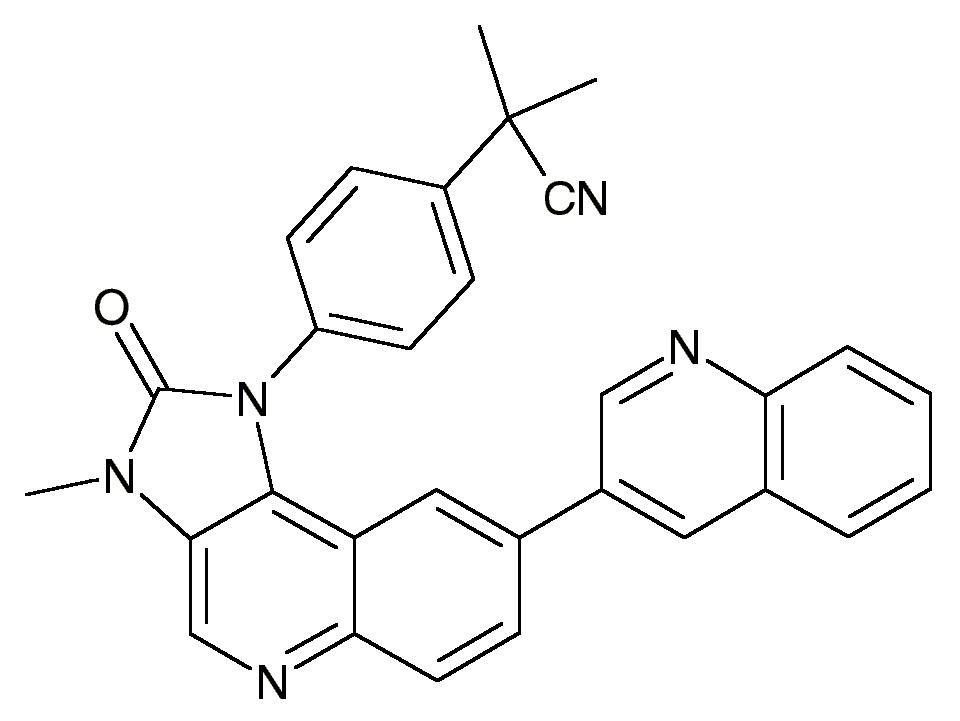
BEZ235 (NVP-BEZ235)Dactolisib
4-[2,3-dihydro-3-methyl-2-oxo-8-(3-quinolinyl)-1H-imidazo[4, 5-c]quinolin-1-yl]-α,α-dimethyl-benzeneacetonitrile
2-methyl-2-{4-[3-methyl-2-oxo-8-(quinolin-3-yl)-1H,2H,3H-imidazo[4,5-c]quinolin-1-yl]phenyl}propanenitrile
2-Methyl-2-[4-(3-methyl-2-oxo-8-quinolin-3-yl-2,3-dihydro-imidazo[4,5-c]quinolin-1-yl)- phenyl]-propionitrile
Chemical Formula: C30H23N5O
CAS Number: 915019-65-7
Molecular Weight: 469.54
PHASE 2, NOVARTIS
CANCER, BLADDER
NVP-BEZ235 is a dual inhibitor of phosphatidylinositol 3-kinase (P13K)and the downstream mammalian target of rapamycin (mTOR) by binding to the ATP-binding cleft of these enzymes. It specifically blocks the dysfunctional activation of the P13K pathway and induce G(1) arrest. NPV-BEZ235 has been shown to inhibit VEGF induced cell proliferation and survival in vitro and VEGF induced angiogenesis in vivo. It has also been shown to inhibit the growth of human cancer in animal models.
BEZ-235 is an orally active phosphatidylinositol 3-kinase (PI3K) inhibitor in early clinical trials at Novartis for the treatment of advanced breast cancer, renal cell carcinoma, solid tumors and castration-resistant prostate cancer. Phase I clinical trials were also under way at the company for the treatment of glioma, however, no developments in this indication has been reported. Phase II clinical trials are ongoing at Johann Wolfgang Goethe Universität for the treatment of relapsed or refractory acute leukemia.
PI3Ks perform various functions, promoting cell growth, proliferation, differentiation, motility, survival and intracellular trafficking. Mutations leading to increased activity of PI3Ks, including faulty production or action of PI3K antagonists, have been found in many cancers.

……………………………..
WO 2006122806
http://www.google.com/patents/WO2006122806A2?cl=en

…………………………..
WO 2008064093
2-methyl-2-[4-(3-methyl- 2-oxo-8-quinolin-3-yl-2,3-dihydro-imidazo[4,5-c]quinolin-1-yl)-phenyl]-propionitrile of formula I (compound I),
Example 1
2-Methyl-2-[4-(3-methyl-2-oxo-8-quinolin-3-yl-2,3-dihydro-imidazo[4,5-c]quinolin-1-yl)- phenyl]-propionitrile
In a suitable lab glass reactor are placed 45.0 g of starting 2[4-(8-bromo-3-methyl-2-oxo-2,3- dihydro-imidazo[4,5-c]quinolin-1-yl)-phenyl]2-methyl-propionitrile together with 2.25 g of bistriphenylphosphine’palladium dichloride in 445 ml N,N-dimethylformamide. This mixture is heated to 95 0C and then a solution of 22.2 g of 3-quinoline boronic acid in a mixture of 225 ml DMF, 300 ml H2O and 60 g of KHCO3 is added. This mixture is heated for 2 h at 95 0C. Then 1080 ml H2O are added. The product 2-methyl-2-[4-(3-methyl-2-oxo-8-quinolin-3-yl- 2,3-dihydro-imidazo[4,5-c]quinolin-1-yl)-phenyl]propionitrile precipitates. The mixture is cooled within 1.5 h to 0 – 5 °C. After stirring at that temperature for 2 h the crude product is filtered and washed with 300 ml H2O. This product is dried in vacuo at 60 0C for 18 h, to yield crude product.
40 g of this crude product is dissolved in 200 ml formic acid at 60 0C. 8 g of active charcoal and Smopex 234 are added. The mixture is stirred at 60 0C for 1 h, the charcoal is filtered, the residue washed with 80 ml formic acid and then 175 ml formic acid are distilled off in vacuo. Then 320 ml methanol are added and the mixture is heated at reflux for 3 h. The purified product precipitates from the reaction mixture. The mixture is cooled to 0 – 5 0C within 1 h, then stirred 2 h at that temperature is finally filtered and washed with 80 ml cold methanol. This recrystallisation procedure is repeated again. Finally the twice recrystallised material is dried in vacuo at 60 0C to yield purified 2-Methyl-2-[4-(3-methyl-2-oxo-8-quinolin- 3-yl-2,3-dihydro-imidazo[4,5-c]quinolin-1-yl)-phenyl]propionitrile.
Example 1a 5-Bromo-2-(2-nitro-vinylamino)-benzoic acid
A suspension of 25 g (16 mmol) of 2-amino-5-bromo-benzoic acid (Fluka, Buchs, Switzerland) in H2O-HCI (37%) (10:1) is stirred for 8 h and then filtered (solution A). 8.17 g (255 mmol) of nitromethane (Fluka, Buchs, Switzerland) are added over 10 min to an ice- bath cooled mixture of 35 g of ice and 15.3 g (382 mmol) of NaOH. After stirring for 1 h at 0 0C and 1 h at rt, the solution is added at 0 0C to 28 g of ice and 42 ml of HCI (37%) (solution B). Solutions A and B are combined and the reaction mixture is stirred for 18 h at rt. The yellow precipitate is filtered off, washed with H2O and dried in vacuo at 400C to give the title compound. ES-MS: 287, 289 (M + H)+, Br pattern; 1H NMR (DMSO-d6): δ 13.7-14.6/br s (1 H), 12.94/d (1 H), 8.07/d (1 H), 8.03/dd (1 H), 7.83/dd (1 H), 7.71/d (1 H), 6.76/d (1 H).
Example 1b 6-Bromo-3-nitro-quinolin-4-ol
29 g (101 mmol) of 5-bromo-2-(2-nitro-vinylamino)-benzoic acid (Example 1a) and 11.9 g (121 mmol) of potassium acetate in 129 ml (152 mmol) of acetic anhydride are stirred for 1.5 h at 120 0C. The precipitate is filtered off and washed with acetic acid until the filtrate is colorless, then is washed with H2O and dried in vacuo to give the title compound. ES-MS: 269, 271 (M + H)+, Br pattern; analytical HPLC: W= 2.70 min (Grad 1).
Example 1c 6-Bromo-4-chloro-3-nitro-quinoline
20 g (74.3 mmol) of 6-bromo-3-nitro-quinolin-4-ol (Example 1b) in 150 ml (1.63 mol) of POCI3 are stirred for 45 min at 120 °C. The mixture is cooled to rt and poured slowly into ice- water. The precipitate is filtered off, washed with ice-cold water, and dissolved in CH2CI2. The organic phase is washed with cold brine, and the aqueous phase is discarded. After drying over MgSO4, the organic solvent is evaporated to dryness to provide the title compound. 1H NMR (CDCI3): J9.20/S (1H), 8.54/d (1H), 8.04/d (1H), 7.96/dd (1H); analytical HPLC: W= 4.32 min (Grad 1).
Example 1d 2-Methyl-2-(4-nitro-phenyl)-propionitrile
O .
To 15 g (92.5 mmol) of (4-nitro-phenyl)-acetonitrile (Fluka, Buchs, Switzerland), 1.64 mg (5.09 mmol) of tetrabutylammonium bromide (Fluka, Buchs, Switzerland) and 43.3 g (305 mmol) of iodomethane in 125 mL of CH2CI2 are added 1O g (250 mmol) of NaOH in 125 ml of water. The reaction mixture is stirred for 20 h at RT. After this time, the organic layer is separated, dried over MgSO4, and evaporated to dryness. The residue is dissolved in diethylether and treated with black charcoal for 30 min, filtered over Celite and evaporated in vacuo to give the title compound as a pale yellow solid. Analytical HPLC: tret= 3.60 minutes (Grad 1).Example 1e (2-(4-Amino-phenyl)-2-methyl-propionitrile
16 g (84.1 mmol) of 2-methyl-2-(4-nitro-phenyl)-propionitrile (Example 1d) and 4.16 g of Raney-Ni are shacked in 160 ml of THF-MeOH (1:1) under 1.1 bar of H2 for 12 h at rt. After completion of the reaction, the catalyst is filtered-off and the filtrate is evaporated to dryness. The residue is purified by flash chromatography on silica gel (hexane-EtOAc 3:1 to 1:2) to provide the title compound as an oil. ES-MS: 161 (M + H)+; analytical HPLC: tret= 2.13 minutes (Grad 1).
Example 1f 2-[4-(6-Bromo-3-nitro-quinolin-4-ylamino)-phenyl]-2-methyl-propionitrile
18 g (62.6 mmol) of 6-bromo-4-chloro-3-nitro-quinoline (Example 1c) and 11 g (68.9 mmol) of (2-(4-amino-phenyl)-2-methyl-propionitrile (Example 1e) are dissolved in 350 ml of acetic acid and stirred for 2 h. After this time, water is added and the yellow precipitate is filtered off and washed with H2O. The solid is dissolved in EtOAc-THF (1 :1), washed with sat. aqueous NaHCO3 and dried over MgSO4. The organic phase is evaporated to dryness to give the title compound as a yellow solid. ES-MS: 411 , 413 (M + H)+, Br pattern; analytical HPLC: tret= 3.69 min (Grad 1).
Example 1q 2-[4-(3-Amino-6-bromo-quinolin-4-ylamino)-phenyl]-2-methyl-propionitrile
24 g (58.4 mmol) of 2-[4-(6-bromo-3-nitro-quinolin-4-ylamino)-phenyl]-2-methyl-propionitrile (Example 1e) is shacked in 300 ml of MeOH-THF (1:1) under 1.1 bar of H2 in the presence of 8.35 g of Raney-Ni for 1 h. After completion of the reaction, the catalyst is filtered off and the filtrate is evaporated to dryness to give the title compound as a yellow foam. ES-MS: 381 , 383 (M + H)+, Br pattern; analytical HPLC: W= 3.21 min (Grad 1).
Example 1h
2-[4-(8-Bromo-2-oxo-2,3-dihydro-imidazo[4,5-c]quinolin-1-yl)-phenyl]-2-methyl- propionitrile
A solution of 5 g (13.1 mmol) of 2-[4-(3-amino-6-bromo-quinolin-4-ylamino)-phenyl]-2- methyl-propionitrile (Example 1g) and 1.59 g (15.7 mmol) of triethylamine in 120 ml CH2CI2 is added over 40 min to a solution of 2.85 g (14.4 mmol) of trichloromethyl chloroformate (Fluka, Buchs, Switzerland) in 80 ml of CH2CI2 at 00C with an ice-bath. The reaction mixture is stirred for 20 min at this temperature then is quenched with sat. aqueous NaHCO3, stirred for 5 min and extracted with CH2CI2. The organic layer is dried over Na2SO4, filtered and evaporated in vacuo to give crude title compound as a brownish solid. ES-MS: 407, 409 (M + H)+, Br pattern; analytical HPLC: tret= 3.05 min (Grad 1). Example 1i
2-[4-(8-Bromo-3-methyl-2-oxo-2,3-dihydro-imidazo[4,5-c]quinolin-1-yl)-phenyl]-2- methyl-propionitrile
To a solution of 3.45 g (8.47 mmol) of 2-[4-(8-bromo-2-oxo-2,3-dihydro-imidazo[4,5- c]quinolin-1-yl)-phenyl]-2-methyl-propionitrile (Example 1h), 1.8 g (12.7 mmol) of iodomethane (Fluka, Buchs, Switzerland) and 273 mg (0.847 mmol) of tetrabutylammonium bromide (Fluka, Buchs, Switzerland) in 170 ml of CH2CI2 is added a solution of 508 mg (12.7 mmol) of NaOH (Fluka, Buchs, Switzerland) in 85 ml of H2O. The reaction mixture is stirred for 2 days and 900 mg (6.35 mmol) of iodomethane and 254 mg (6.35 mmol) of NaOH in 5 ml of H2O are added. The reaction mixture is stirred for 1 day at rt . After this time, the reaction is quenched with H2O and extracted with CH2CI2 (2*). The organic layer is washed with brine, dried over Na2SO4, filtered and evaporated in vacuo to give the title compound as a beige solid. ES-MS: 421 , 423 (M + H)+, Br pattern; analytical HPLC: tret= 3.15 min (Grad 1).
Example 2
2-Methyl-2-[4-(3-methyl-2-oxo-8-quinolin-3-yl-2,3-dihydro-imidazo[4,5-c]quinolin-1-yl)- phenyl]propionitrile p-toluenesulfonate salt
26.5 g of 2-Methyl-2-[4-(3-methyl-2-oxo-8-quinolin-3-yl-2,3-dihydro-imidazo[4,5-c]quinolin-1- yl)-phenyl]propionitrile are placed together with 55 ml formic acid into a glass reactor. This mixture is heated to 60 0C to get a clear solution. This solution is clearfiltered and washed with 36 ml formic acid. Then formic acid is distilled off until the volume of the residual solution is 55 ml. Then a solution of 11.3 g of p-toluenesulfonic acid in 228 ml acetone is added at 50 0C, followed by further addition of 822 ml acetone within 30 minutes. The salt precipitates from the reaction mixture. The mixture is cooled to 0 0C within 2 h, stirred at that temperature for 3 h, is then filtered and washed with 84 ml acetone. The product‘ is dried at 60 0C in vacuo for 18 h to yield 29.8 g (82.4 %) of the 2-Methyl-2-[4-(3-methyl-2-oxo-8- quinolin-3-yl-2,3-dihydro-imidazo[4,5-c]quinolin-1-yl)-phenyl]propionitrile p-toluenesulfonate salt (crystalline form A). The crystalline forms of the present invention are synthesized in accordance with the following examples which are illustrative without limiting the scope of the present invention.
Example 3:
Preparation of form A of 2-methyl-2-[4-(3-methyl-2-oxo-8-quinolin-3-yl-2,3-dihydro- imidazo[4,5-c]quinolin-1-yl)-phenyl]-propionitrile
Form A of compound I can be manufactured in the following way: 241 g of free base are dissolved 2.4 I acetic acid at 50 0C. The solution is clearfiltered, washed with 250 ml acetic acid and then at 50 0C 7.2 I of water are added. The free base starts precipitating. The mixture is cooled within 1 h to 25 0C, is then filtered and washed with 10 I H2O. The free base is then dried in vacuo at 50 0C over night to yield 204 g of free base.
References
| WO2005054237A1 | 19 Nov 2004 | 16 Jun 2005 | Hans-Georg Capraro | 1h-imidazoquinoline derivatives as protein kinase inhibitors |
| WO2006122806A2 | 18 May 2006 | 23 Nov 2006 | Novartis Ag | 1,3-dihydro-imidazo [4,5-c] quinolin-2-ones as lipid kinase inhibitors |
| CL11872006A | Title not available |
| Citing Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| WO2009118324A1 * | 24 Mar 2009 | 1 Oct 2009 | Novartis Ag | 5imidazoquinolines and pyrimidine derivatives as potent modulators of vegf-driven angiogenic processes |
| WO2013049300A1 * | 27 Sep 2012 | 4 Apr 2013 | Dana-Farber Cancer Institute, Inc. | Method of treating mucoepidermoid carcinoma |
| WO2013152717A1 | 9 Apr 2013 | 17 Oct 2013 | Shanghai Yunyi Healthcare Management Co., Ltd. | Fused pyrimidine compound, and preparation method, intermediate, composition, and uses thereof |
| EP2474323A2 * | 24 Mar 2009 | 11 Jul 2012 | Novartis AG | Imidazoquinolines and pyrimidine derivatives as potent modulators of vegf-driven angiogenic processes |
| US8476294 | 2 Jun 2010 | 2 Jul 2013 | Novartis Ag | 1H-imidazo[4,5-c]quinolinone derivatives |
Filed under: cancer, Phase2 drugs, Uncategorized Tagged: BEZ235, BLADDER, Dactolisib, NOVARTIS CANCER, NPV-BEZ235, NVP-BEZ235, phase 2