

Tucidinostat, Chidamide
ツシジノスタット
2021/6/23 PMDA JAPAN APPROVED,
| Hiyasta |
| Formula | C22H19FN4O2 |
|---|---|
| CAS | 1616493-44-7 |
| Mol weight | 390.4103 |
Antineoplastic, Histone deacetylase inhibitor
Chidamide (Epidaza) is a histone deacetylase inhibitor (HDI) developed in China.[1] It was also known as HBI-8000.[2] It is a benzamide HDI and inhibits Class I HDAC1, HDAC2, HDAC3, as well as Class IIb HDAC10.[3]
Chidamide is approved by the Chinese FDA for relapsed or refractory peripheral T-cell lymphoma (PTCL), and has orphan drug status in Japan.[2][better source needed] As of April 2015 it is only approved in China.[1]
Chidamide is being researched as a treatment for pancreatic cancer.[4][5][6] However, it is not US FDA approved for the treatment of pancreatic cancer.
Chidamide (Epidaza®), a class I HDAC inhibitor, was discovered and developed by ChipScreen and approved by the CFDA in December 2014 for the treatment of recurrent of refractory peripheral T-cell lymphoma. Chidamide, also known as CS055 and HBI- 8000, is an orally bioavailable benzamide type inhibitor of HDAC isoenzymes class I 1–3, as well as class IIb 10, with potential antineoplastic activity. It selectively binds to and inhibits HDAC, leading to an increase in acetylation levels of histone protein H3.74 This agent also inhibits the expression of signaling kinases in the PI3K/ Akt and MAPK/Ras pathways and may result in cell cycle arrest and the induction of tumor cell apoptosis. Currently, phases I and II clinical trials are underway for the treatment of non-small cell lung cancer and for the treatment of breast cancer, respectively.
Chemical Synthesis
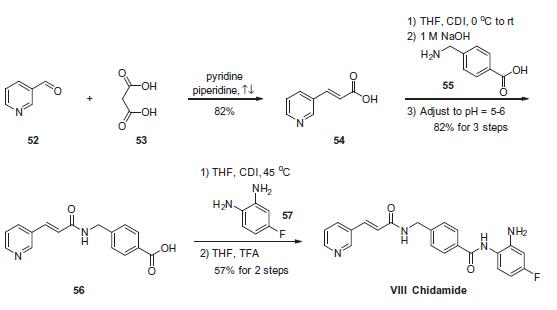
The scalable synthetic approach to chidamide very closely follows the discovery route. The sequence began with the condensation of commercial nicotinaldehyde (52) and malonic acid (53) in a mixture of pyridine and piperidine. Next, activation of acid 54 with N,N0-carbonyldiimidazole (CDI) and subsequent reaction with 4-aminomethyl benzoic acid (55) under basic conditions afforded amide 56 in 82% yield. Finally, activation of 56 with CDI prior to treatment with 4-fluorobenzene- 1,2-diamine (57) and subsequent treatment with TFA and THF yielded chidamide (VIII) in 38% overall yield from 52. However, no publication reported that mono-N-Boc-protected bis-aniline was used to approach Chidamide.
References
- ^ Jump up to:a b Lowe D (April 2015). “China’s First Homegrown Pharma”. Seeking Alpha.
- ^ Jump up to:a b “Chipscreen Biosciences Announces CFDA Approval of Chidamide (Epidaza) for PTCLs in China”. PR Newswire Association LLC.
- ^ “HUYA Bioscience International Grants An Exclusive License For HBI-8000 In Japan And Other Asian Countries To Eisai”. PR Newswire Association LLC. February 2016.
- ^ Qiao Z, Ren S, Li W, Wang X, He M, Guo Y, et al. (April 2013). “Chidamide, a novel histone deacetylase inhibitor, synergistically enhances gemcitabine cytotoxicity in pancreatic cancer cells”. Biochemical and Biophysical Research Communications. 434 (1): 95–101. doi:10.1016/j.bbrc.2013.03.059. PMID 23541946.
- ^ Guha M (April 2015). “HDAC inhibitors still need a home run, despite recent approval”. Nature Reviews. Drug Discovery. 14 (4): 225–6. doi:10.1038/nrd4583. PMID 25829268. S2CID 36758974.
- ^ Wang SS (2015-04-02). “A New Cancer Drug, Made in China”. The Wall Street Journal. Retrieved 13 April 2015.
| Clinical data | |
|---|---|
| Trade names | Epidaza |
| Other names | Tucidinostat |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 1616493-44-7 |
| PubChem CID | 9800555 |
| ChemSpider | 7976319 |
| UNII | 87CIC980Y0 |
| Chemical and physical data | |
| Formula | C22H19FN4O2 |
| Molar mass | 390.418 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| showSMILES | |
| showInChI |
/////Tucidinostat, Antineoplastic, Histone deacetylase inhibitor, ツシジノスタット , Epidaza, Chidamide, APPROVALS 2021, JAPAN 2021
