

Sinovac COVID-19 vaccine, CoronaVac,
- PiCoVacc
CoronaVac, also known as the Sinovac COVID-19 vaccine,[1] is an inactivated virus COVID-19 vaccine developed by the Chinese company Sinovac Biotech.[2] It has been in Phase III clinical trials in Brazil,[3] Chile,[4] Indonesia,[5] the Philippines,[6] and Turkey.[7]
It relies on traditional technology similar to BBIBP-CorV and BBV152, other inactivated-virus COVID-19 vaccines in Phase III trials.[8] CoronaVac does not need to be frozen, and both the vaccine and raw material for formulating the new doses could be transported and refrigerated at 2–8 °C (36–46 °F), temperatures at which flu vaccines are kept.[9]
Brazil announced results on 13 January 2021 showing 50.4% effective at preventing symptomatic infections, 78% effective in preventing mild cases needing treatment, and 100% effective in preventing severe cases.[10] Final Phase III results from Turkey announced on 3 March 2021 showed an efficacy of 83.5%.[11] Interim results in Indonesia were announced on 11 January 2021 with an efficacy of 65.3%.[12] A detailed report containing confidence intervals, efficacy by age and side effects has not yet been released.
CoronaVac is being used in vaccination campaigns by certain countries in Asia,[13][14][15] South America,[16][17][18] North America,[19][20] and Europe.[21] In March, a Sinovac spokesman told Reuters production capacity for CoronaVac could reach 2 billion doses a year by June 2021.[22] As of March 21, 70 million doses of CoronaVac had been administered worldwide.[23
Technology
CoronaVac is an inactivated vaccine. It uses a similar, more traditional technology as in BBIBP-CorV and BBV152, other inactivated-virus vaccines for COVID-19 in Phase III trials.[24][25] CoronaVac does not need to be frozen, and both the vaccine and raw material for formulating the new doses could be transported and refrigerated at 2–8 °C (36–46 °F), temperatures at which flu vaccines are kept.[26] CoronaVac could remain stable for up to three years in storage, which might offer some advantage in vaccine distribution to regions where cold chains are not developed.[27]
Efficacy

Empty bottle of CoronaVac
On 7 January 2021, results from Phase III trials in Brazil among 13,000 volunteers revealed the vaccine was 78% effective in preventing symptomatic cases of COVID-19 requiring medical assistance (grade 3 on the WHO Clinical Progression Scale[28]) and 100% effective against moderate and severe infections.[29] After mounting pressure from scientists, Butantan said on 12 January that these rates only included volunteers who had mild to severe cases of COVID-19.[30] The overall efficacy, including asymptomatic cases and symptomatic cases not requiring medical assistance (WHO grade 2), was 50.38%.[31] Of the 220 participants infected, 160 cases were in the placebo group and 60 cases in the group that received CoronaVac.[32]
On 3 March 2021, final Phase III results from Turkey showed an efficacy of 83.5%. The final efficacy rate was based on 41 infections, 32 of which had received a placebo, said Murat Akova, head of the Phase III trials in Turkey. He added the vaccine prevented hospitalization and severe illness in 100% of cases, saying six people who were hospitalized were all in the placebo group. The final results were based on a 10,216 participants, 6,648 of whom received the vaccine as part of the Phase III study that began mid-September. Turkey had announced an interim result with 29 infections in December, which placed the efficacy at 91.25%.[33][34]
On 11 January, Indonesia released Phase III results from an interim analysis of 25 cases which showed an efficacy rate of 65.3% based on data of 1,600 participants in the trial.[35] The trial was conducted in the city of Bandung, and it was not clear how Indonesian scientists made their calculations.[30]
Variability in results
Officials said the lowered figure of 50.4% included “very light” cases of COVID-19 among participants omitted in the earlier analysis. Ricardo Palácios, Medical Director of Instituto Butantan said Sinovac’s relatively low efficacy rate of 50% was due to more rigorous standards for what counts as an infection among trial participants. The Institute included six types of cases in its results: asymptomatic, very mild, mild, two levels of moderate, and severe, while western vaccine makers generally included only mild, moderate, and severe categories. Brazil’s trial was also largely made up of frontline health care workers. “They are more exposed to the virus and may explain the relatively low efficacy rate,” said Yanzhong Huang, a senior fellow for global health at the Council on Foreign Relations.[36]
The release of more definitive data on CoronaVac’s efficacy was delayed because Sinovac needed to reconcile results from different trials using varying protocols.[32] According to Instituto Butantan director Dimas Covas, the Brazilian group was considered more vulnerable to infection and exposure to higher viral loads. In Turkish and Indonesian Phase III trials, the composition of volunteers was similar to that of the general population.[37]
COVID-19 variants
On March 10, Instituto Butantan Director Dimas Covas said CoronaVac was efficient against three variants of COVID-19 in the country; British B.1.1.7, South African 501.V2, and Brazil’s P.1, of which are derived variants P.1 from Manaus state, and P.2 from Rio de Janeiro.[38]
CoronaVac and other inactivated virus vaccines have all parts of the virus. Butantan said this may generate a more comprehensive immune response compared to other vaccines using only a part of the spike protein used by COVID-19 to infect cells. Tests run by Butantan used the serum of vaccinated people, which are placed in a cell culture and subsequently infected with the variants. The neutralization consists of determining whether antibodies generated from the vaccine will neutralize the virus in the culture.[38]
Clinical trials
For broader coverage of this topic, see COVID-19 vaccine.
Phase I–II
In a Phase II clinical trial completed in July 2020 and published in The Lancet, CoronaVac showed seroconversion of neutralising antibodies for 109 (92%) of 118 participants in the 3 μg group, 117 (98%) of 119 in the 6 μg group, after the days 0 and 14 schedule; whereas at day 28 after the days 0 and 28 schedule, seroconversion was seen in 114 (97%) of 117 in the 3 μg group, 118 (100%) of 118 in the 6 μg group.[39]
In May, CoronaVac began Phase I–II trials in China on adults over the age 60, and in September CoronaVac began Phase I–II trials in China on children ages 3–17.[40] Phase II results for older adults published in The Lancet showed CoronaVac was safe and well tolerated in older adults, with neutralising antibody induced by a 3 μg dose were similar to those of a 6 μg dose.[41]
Phase III
Latin America
In late July 2020, Sinovac began conducting a Phase III vaccine trial to evaluate efficacy and safety on 9,000 volunteer healthcare professionals in Brazil, collaborating with Butantan Institute.[42][43] On 19 October, São Paulo Governor João Doria said the first results of the clinical study conducted in Brazil proved that among the vaccines being tested in the country, CoronaVac is the safest, the one with the best and most promising immunization rates.[44] On 23 October, São Paulo announced the creation of six new centers for trials of CoronaVac, increasing the number of volunteers in the trials to 13,000.[45]
Brazil briefly paused Phase III trials on 10 November after the suicide of a volunteer before resuming on 11 November. Instituto Butantan said the suicide had no relation to the vaccine trial.[46][47]
In August, a Phase III trial was started in Chile, headed by Pontifical Catholic University of Chile, which was expected to include 3,000 volunteers between the ages of 18 and 65.[48]
Europe
In September, Turkey began Phase III trials with 13,000 volunteers on a two-dose 14-day interval.[49] The monitoring process for CoronaVac is underway at 25 centers in 12 cities across the country.[50]

The Governor of West Java Ridwan Kamil participating in phase 3 trial of the Sinovac COVID-19 vaccine in Indonesia.
Asia
In August, Sinovac began Phase III trials in Indonesia with Bio Farma in Bandung involving 1,620 volunteers.[51] In November, Padjadjaran University Medical School provided an update that the trials were running smoothly and that “at most, they found a slight body fever which disappeared within two days”.[52]
In October, Saudi Arabia signed an agreement with Sinovac to distribute CoronaVac to 7,000 healthcare workers, after conducting Phase III trials with the Saudi Arabian National Guard.[53]
Manufacturing
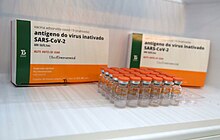
Brazilian version of CoronaVac, manufactured by Butantan
In March, a Sinovac spokesman told Reuters production capacity for CoronaVac could reach 2 billion doses a year by June. The figure is double the capacity of 1 billion doses in bulk ingredients the firm said it could reach by February.[22]
After Indonesia’s Phase III trials, Bio Farma plans to ramp up production to 250 million doses a year.[54]
On 9 November, São Paulo began building a facility to produce 100 million doses a year.[55] On 10 December, João Doria said Butantan aimed to fill and finish 1 million doses per day on its production line for a vaccination campaign starting 25 January. Doria said 11 Brazilian states have contacted Butantan seeking doses of CoronaVac.[56]
In Malaysia, Pharmaniaga will manufacture, fill, and finish CoronaVac. Pharmaniaga signed a deal to obtain bulk supply of the vaccine as well as technology transfer from Sinovac.[57]
In Egypt, the government was in “advanced stage” discussions with Sinovac to manufacture CoronaVac for local use and export to African countries.[58]
Market and deployment
As of March 21, 70 million doses of CoronaVac had been administered worldwide.[23]
| show Full authorizationshow Emergency authorization Eligible COVAX recipient (assessment in progress)[80] |
South America

São Paulo State Secretary of Health Jean Gorinchteyn (left) and Instituto Butantan chairman Dimas Covas (right) holding single-dose prefilled syringes of CoronaVac, part of the fourth shipment of Sinovac-manufactured vaccine to arrive in Brazil
In Brazil, São Paulo governor João Doria signed a $90 million contract with Sinovac in September to receive the initial 46 million doses of CoronaVac.[81] The price for CoronaVac was announced to be US$10.3 (about R$59).[82] In January, Brazil announced it would obtain 100 million total doses.[83] On 17 January, ANVISA approved emergency use of CoronaVac, with a 54-year-old nurse in São Paulo being the first to receive a vaccine outside of clinical trials in the country.[16] In early February, Brazil said it intends to buy an additional 30 million doses to be produced locally on top of the existing 100 million doses.[84]
In January, Bolivia authorized use of CoronaVac. Butantan Institute had opened negotiations with South American countries to sell the vaccine, which would be produced in São Paulo.[85]
In October, Chile signed an agreement to purchase 20 million doses of CoronaVac[86] which was approved for emergency use on 20 January.[87] By early March, the country had received 10 million doses of CoronaVac and had vaccinated 4.1 million people.[88]
In February, Colombia had purchased 5 million doses of CoronaVac and was in talks for an additional 5 million doses,[89] which had been approved for emergency use on February 5.[90]
In February, Ecuador signed a deal for 2 million doses of CoronaVac which had been approved for emergency use.[91] Chile donated 20,000 doses of CoronaVac to Ecuador on March 6.[92]
In March, Paraguay received a donation of 20,000 doses of CoronaVac from Chile.[92] Paraguay began vaccinations with CoronaVac on March 10.[93]
In January, Uruguay announced the purchased of 1.75 million doses of CoronaVac.[94] The first 192,000 doses arrived on 25 February and vaccinations started on 1 March.[18]
Europe
In March, Albania received 192,000 doses of a first batch of 1 million doses purchased through Turkey.[95]
In November, Turkey signed a contract to buy 50 million doses of CoronaVac.[96] Turkey approved emergency use on 13 January[97] and President Recep Tayyip Erdoğan received his first dose at Ankara City Hospital.[98] In February, Turkey signed a deal for another 50 million doses for a total of 100 million doses.[21] By March 10.7 million doses had been administered, and 852 of the 1.3 million people who had received both doses were later diagnosed with the disease. 53 were hospitalized, but none of those hospitalized were intubated or died.[99]
In December, Ukraine signed a contract to purchase 1.8 million doses of CoronaVac. One dose of CoronaVac would cost 504 hryvnias (around $18).[100] On March 9, Ukraine granted approval for use of CoronaVac.[101]
Asia
On 19 January, Azerbaijan launched its vaccination campaign with CoronaVac. Azerbaijan plans to receive 4 million doses of the vaccine and aims to vaccinate 40% of the population.[102]
In February, Cambodia approved Coronavac[103] for emergency use and later ordered 1.5 million doses to arrive on March 26.[104]
In late August, China approved CoronaVac for emergency use to vaccinate high-risk groups such as medical staff.[105] In early February, China approved CoronaVac for general use.[15]
In December, Hong Kong ordered 7.5 million doses of CoronaVac.[106] The vaccination campaign with CoronaVac began on 26 February.[107]
In August, Indonesia’s Foreign Minister Retno Marsudi said an agreement was signed with Sinovac for 50 million doses,[108] which later increased to 140 million doses.[109] Indonesia approved emergency use authorization on 11 January and[35] President Joko Widodo received the first shot of the vaccine, which would be free for all Indonesian citizens.[13] By March, Indonesia had received 53.5 million doses of CoronaVac.[110]
On 26 January, Malaysia ordered 12 million doses.[57] CoronaVac was approved for emergency use on 2 March.[111] Malaysian Science, Technology and Innovation Minister Khairy Jamaluddin received the first dose with CoronaVac on 18 March as part of the vaccination campaign.[112]
In January, the Philippine’s announced the country had secured 25 million doses.[113] The vaccine was approved on 22 February but not for all health workers as it had lower efficacy when used with health workers compared to healthy individuals aged 18-59. The first 600,000 doses of CoronaVac arrived on 28 February.[114]
Singapore has signed advance purchase agreements for CoronaVac.[115] In February, the first doses arrived in the country.[116]
In early January, Thailand’s Ministry of Public Health announced an order for 2 million doses of CoronaVac,[117] which was approved for emergency use on 22 February.[118] Thailand started its vaccination program on 27 February.[14] In March, Thailand was in talks to purchase an additional 5 million doses.[119]
North America
By March 8, Dominican Republic had vaccinated 400,000 people and had reserved delivery for 10 million additional doses of CoronaVac.[19]
In February, Mexico approved emergency use of CoronaVac.[120] The country has ordered 20 million doses,[121] of which the first 200,000 doses arrived on 20 February.[122] It is currently used as part of the national vaccination campaign.[20]
Africa
In March, Benin received 203,000 doses of CoronaVac with vaccinations to start with health workers and the medically vulnerable.[123]
In March, South Africa’s drug regulator began assessing CoronaVac for use in the country.[124] South African firm Numolux said it could supply 5 million doses once it secured regulatory clearances.[125]
In March, Tunisia’s Ministry of Health approved marketing authorization of CoronaVac in the country.[126]
In March, Zimbabwe approved CoronaVac for emergency use.[127]
Oceania
In March, Fiji said it would be receiving a donation of CoronaVac.[128]
Controversies
Politicization
CoronaVac has been championed by the governor of São Paulo, João Doria, who many believe will challenge Jair Bolsonaro for the presidency in 2022.[129] A political showdown began in October 2020, when Bolsonaro vetoed a deal between the Brazilian health ministry and the São Paulo government for the purchase of 46 million doses of the vaccine.[130] After Instituto Butantan announced CoronaVac’s efficacy rate, Bolsonaro mocked the vaccine’s effectiveness against COVID-19.[131] Critics against the politicization of vaccines have warned that failure to follow international testing and safety protocols risks undermining public trust and can increase people’s hesitancy to inoculation.[129] Doctors in São Paulo said they were struggling to convince patients that CoronaVac would be safe.[132]
In March 2021, the Paraná Pesquisas opinion polling institute found that the vaccines preferred by Brazilians are CoronaVac and the Oxford–AstraZeneca vaccine, chosen by 23.6% and 21.2% of Brazilians interviewed, respectively, against 11.3% of those who would prefer the Pfizer–BioNTech vaccine.[133]
Delays in releasing results
On 23 December 2020, researchers in Brazil said the vaccine was more than 50% effective, but withheld full results at Sinovac’s request, raising questions again about transparency as it was the third delay in releasing results from the trials.[134] São Paulo Health Secretary Jean Gorinchteyn later said the vaccine didn’t reach 90% efficacy. Turkey said its trial showed an estimated efficacy rate of 91.25%, though that was based on only 29 infected cases.[32] When São Paulo state officials announced the protection rate, they declined to provide a more detailed breakdown of the trial, such as information about age groups and side effects of the vaccine.[32] Scientists said the lack of transparency about the data ran the risk of damaging CoronaVac’s credibility, with Brazilians and others world-wide already reluctant to take it.[30] Nikolai Petrovsky, a professor at the College of Medicine and Public Health at Flinders University said, “There is enormous financial and prestige pressure for these trials to massively overstate their results.”[135]
References
- ^ Corum, Jonathan; Zimmer, Carl. “How the Sinovac Vaccine Works”. The New York Times. ISSN 0362-4331. Retrieved 1 March 2021.
- ^ Nidhi Parekh (22 July 2020). “CoronaVac: A COVID-19 Vaccine Made From Inactivated SARS-CoV-2 Virus”. Retrieved 25 July2020.
- ^ “New coronavirus vaccine trials start in Brazil”. AP News. 21 July 2020. Retrieved 7 October 2020.
- ^ “Chile initiates clinical study for COVID-19 vaccine”. Chile Reports. 4 August 2020. Retrieved 7 October 2020.
- ^ “248 volunteers have received Sinovac vaccine injections in Bandung”. Antara News. 30 August 2020. Retrieved 7 October2020.
- ^ “DOH eyes 5 hospitals for Sinovac vaccine Phase 3 clinical trial”. PTV News. 16 September 2020. Retrieved 7 October 2020.
- ^ “Turkey begins phase three trials of Chinese Covid-19 vaccine”. TRT World News. 1 September 2020. Retrieved 7 October 2020.
- ^ Zimmer, Carl; Corum, Jonathan; Wee, Sui-Lee. “Coronavirus Vaccine Tracker”. The New York Times. ISSN 0362-4331. Retrieved 12 February 2021.
- ^ “CoronaVac: Doses will come from China on nine flights and can…” AlKhaleej Today (in Arabic). 1 November 2020. Retrieved 12 February 2021.
- ^ “Sinovac: Brazil results show Chinese vaccine 50.4% effective”. BBC News. 13 January 2021. Retrieved 12 February 2021.
- ^ AGENCIES, DAILY SABAH WITH (25 December 2020). “Turkey set to receive ‘effective’ COVID-19 vaccine amid calls for inoculation”. Daily Sabah. Retrieved 12 February 2021.
- ^ hermesauto (11 January 2021). “Indonesia grants emergency use approval to Sinovac’s vaccine, local trials show 65% efficacy”. The Straits Times. Retrieved 12 February 2021.
- ^ Jump up to:a b TARIGAN, EDNA; MILKO, VICTORIA (13 January 2021). “Indonesia starts mass COVID vaccinations over vast territory”. Associated Press. Retrieved 15 January 2021.
- ^ Jump up to:a b “Thailand Kicks Off Covid-19 Vaccine Program With Sinovac Shots”. Bloomberg.com. Retrieved 28 February 2021.
- ^ Jump up to:a b “China approves Sinovac vaccines for general public use”. South China Morning Post. 6 February 2021. Retrieved 6 February2021.
- ^ Jump up to:a b Fonseca, Jamie McGeever, Pedro (17 January 2021). “Brazil clears emergency use of Sinovac, AstraZeneca vaccines, shots begin”. Reuters. Retrieved 17 January 2021.
- ^ Miranda, Natalia A. Ramos (28 January 2021). “Chile receives two million-dose first delivery of Sinovac COVID-19 vaccine”. Reuters. Retrieved 30 January 2021.
- ^ Jump up to:a b “BNamericas – Uruguay prepares to launch COVID-19 vaccinat…” BNamericas.com. Retrieved 1 March 2021.
- ^ Jump up to:a b “Anticovid vaccines run out as Dominican Republic awaits arrival of more doses”. Dominican Today. Retrieved 10 March2021.
- ^ Jump up to:a b “Venustiano Carranza next up for Covid vaccination in Mexico City”. Mexico News Daily. 15 March 2021. Retrieved 16 March2021.
- ^ Jump up to:a b “Turkey aims to vaccinate 60 percent of population: Minister – Turkey News”. Hürriyet Daily News. Retrieved 12 February 2021.
- ^ Jump up to:a b Liu, Roxanne (3 March 2021). “Sinovac eyes two billion doses in annual capacity of virus vaccine by June”. Reuters. Retrieved 3 March 2021.
- ^ Jump up to:a b Liu, Roxanne (21 March 2021). “China steps up COVID-19 vaccination, considers differentiated visa policies”. Reuters. Retrieved 21 March 2021.
- ^ Tan Y (16 December 2020). “Covid: What do we know about China’s coronavirus vaccines?”. BBC News. Retrieved 18 December 2020.
- ^ Zimmer C, Corum J, Wee SL (10 June 2020). “Coronavirus Vaccine Tracker”. The New York Times. ISSN 0362-4331. Retrieved 27 December 2020.
- ^ “CoronaVac: Doses will come from China on nine flights and can…” AlKhaleej Today (in Arabic). 1 November 2020. Archivedfrom the original on 16 December 2020. Retrieved 1 November2020.
- ^ Staff (7 September 2020). “China’s Sinovac coronavirus vaccine candidate appears safe, slightly weaker in elderly”. Reuters. Archived from the original on 7 October 2020. Retrieved 6 October 2020.
- ^ WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection (2020). “A minimal common outcome measure set for COVID-19 clinical research”. The Lancet Infectious Diseases. 20 (8): e192–e197. doi:10.1016/S1473-3099(20)30483-7. PMC 7292605. PMID 32539990.
- ^ Mariz, Fabiana; Caires, Luiza (7 January 2021). “Eficaz em prevenir doença grave e morte por covid, Coronavac deve ter impacto em frear pandemia”. Jornal da USP (in Portuguese). Retrieved 7 January 2021.
- ^ Jump up to:a b c Pearson, Samantha; Magalhaes, Luciana (12 January 2021). “Chinese Covid-19 Vaccine Is Far Less Effective Than Initially Touted in Brazil”. The Wall Street Journal. Retrieved 12 January2021.
- ^ Gielow, Igor; Lopes Batista, Everton; Bottallo, Ana (12 January 2021). “Coronavac tem eficácia geral de 50,38% no estudo feito pelo Butantan”. Folha de S. Paulo (in Portuguese). Retrieved 12 January 2021.
- ^ Jump up to:a b c d “Sinovac’s Covid Shot Proves 78% Effective in Brazil Trial”. Bloomberg L.P. 7 January 2021. Retrieved 7 January 2021.
- ^ Kucukgocmen, Ali (3 March 2021). “Turkish study revises down Sinovac COVID-19 vaccine efficacy to 83.5%”. Reuters. Retrieved 7 March 2021.
- ^ “China’s Sinovac vaccine efficacy 83.5 percent: Turkish researchers – Turkey News”. Hürriyet Daily News. Retrieved 7 March 2021.
- ^ Jump up to:a b hermesauto (11 January 2021). “Indonesia grants emergency use approval to Sinovac’s vaccine, local trials show 65% efficacy”. The Straits Times. Retrieved 11 January 2021.
- ^ “Why did the efficacy of China’s top vaccine drop from 78% to 50%?”. Fortune. Retrieved 14 January 2021.
- ^ “Coronavac tem eficácia de 78% contra a Covid-19 em estudo no Brasil”. Folha de S.Paulo (in Portuguese). 7 January 2021. Retrieved 7 January 2021.
- ^ Jump up to:a b “Estudos mostram eficácia da CoronaVac contra três variantes do vírus”. Agência Brasil (in Portuguese). 10 March 2021. Retrieved 18 March 2021.
- ^ Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, et al. (November 2020). “Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial”. The Lancet. Infectious Diseases. 21 (2): 181–192. doi:10.1016/S1473-3099(20)30843-4. PMC 7832443. PMID 33217362. S2CID 227099817. Archived from the original on 16 December 2020. Retrieved 18 November 2020.
- ^ Clinical trial number NCT04551547 for “A Randomized, Double-Blinded, Placebo-Controlled, Phase I/II Clinical Trial, to Evaluate the Safety and Immunogenicity of the SARS-CoV-2 Inactivated Vaccine (Vero Cell) in Healthy Population Aged 3–17 Years” at ClinicalTrials.gov
- ^ Wu, Zhiwei; Hu, Yaling; Xu, Miao; Chen, Zhen; Yang, Wanqi; Jiang, Zhiwei; Li, Minjie; Jin, Hui; Cui, Guoliang; Chen, Panpan; Wang, Lei (3 February 2021). “Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial”. The Lancet Infectious Diseases. 0. doi:10.1016/S1473-3099(20)30987-7. ISSN 1473-3099. PMC 7906628. PMID 33548194.
- ^ Savarese M (21 July 2020). “New coronavirus vaccine trials start in Brazil”. Associated Press. Archived from the original on 13 August 2020. Retrieved 15 August 2020.
- ^ Palacios R, Patiño EG, de Oliveira Piorelli R, Conde MT, Batista AP, Zeng G, et al. (October 2020). “Double-Blind, Randomized, Placebo-Controlled Phase III Clinical Trial to Evaluate the Efficacy and Safety of treating Healthcare Professionals with the Adsorbed COVID-19 (Inactivated) Vaccine Manufactured by Sinovac – PROFISCOV: A structured summary of a study protocol for a randomised controlled trial”. Trials. 21 (1): 853. doi:10.1186/s13063-020-04775-4. PMC 7558252. PMID 33059771. Archived from the original on 16 December 2020. Retrieved 28 October 2020.
- ^ “World’s vaccine testing ground deems Chinese COVID candidate ‘the safest, most promising'”. Fortune. Archived from the original on 31 October 2020. Retrieved 9 November 2020.
- ^ “Doria says it guarantees purchase of 100 million doses of CoronaVac…” AlKhaleej Today (in Arabic). 29 October 2020. Archived from the original on 1 November 2020. Retrieved 30 October 2020.
- ^ “Brazil Clears Sinovac Trial to Resume Two Days After Halting It”. Bloomberg L.P. 11 November 2020. Archived from the original on 11 November 2020. Retrieved 11 November 2020.
- ^ “Brazil’s health regulator says China’s Sinovac can resume Covid-19 vaccine trial after suspension”. CNBC. 12 November 2020. Archived from the original on 13 November 2020. Retrieved 17 November 2020.
- ^ “Chile initiates clinical study for COVID-19 vaccine”. Government of Chile. 4 August 2020. Archived from the original on 11 October 2020. Retrieved 28 August 2020.
- ^ Health Institutes of Turkey (8 October 2020). “Randomized, Double-Blind, Placebo-Controlled Phase III Clinical Trial For Evaluation of Efficacy and Safety of SARS-CoV-2 Vaccine (Vero Cell), Inactivated”. ClinicalTrials. Archived from the original on 20 October 2020. Retrieved 21 October 2020.
- ^ “Chinese COVID-19 vaccine to be free, 1st doses to be delivered soon: Turkey’s health minister”. Daily Sabah. 23 November 2020. Archived from the original on 23 November 2020. Retrieved 23 November 2020.
- ^ “248 volunteers have received Sinovac vaccine injections in Bandung”. Antara News. Archived from the original on 30 September 2020. Retrieved 22 September 2020.
- ^ antaranews.com. “Phase 3 Sinovac clinical trial running smoothly: research team”. Antara News. Archived from the original on 3 November 2020. Retrieved 3 November 2020.
- ^ “Virus vaccine waiting on Saudi ‘green light'”. Arab News. 31 October 2020. Archived from the original on 16 December 2020. Retrieved 1 November 2020.
- ^ hermesauto (12 October 2020). “Indonesia aims to start administering coronavirus vaccines in early November”. The Straits Times. Archived from the original on 13 October 2020. Retrieved 12 October 2020.
- ^ “Sao Paulo starts building production plant for China’s Sinovac vaccine – governor”. Financial Post. Archived from the original on 29 November 2020. Retrieved 9 November 2020.
- ^ Mano A, Simões (10 December 2020). “Chinese vaccine draws demand across Latin America, say Brazilian officials”. Reuters. Archived from the original on 10 December 2020. Retrieved 10 December 2020.
- ^ Jump up to:a b Choong, Jerry (26 January 2021). “Health Ministry: Malaysia secures 18.4 million doses of Russian, Chinese Covid-19 vaccines”. The Malay Mail. Retrieved 26 January 2021.
- ^ Mourad, Mahmoud (22 March 2021). “Egypt aims for deal to produce Sinovac COVID-19 vaccines”. Reuters. Retrieved 22 March 2021.
- ^ Jump up to:a b c Liu R (6 February 2021). “China approves Sinovac Biotech COVID-19 vaccine for general public use”. Reuters. Retrieved 7 February 2021.
- ^ Sipalan, Joseph; Donovan, Kirsten (3 March 2021). “Malaysia approves Sinovac, AstraZeneca COVID-19 vaccines for use”. Reuters. Retrieved 7 March 2021.
- ^ Aliyev J. “Azerbaijan kicks off COVID-19 vaccination”. Anadolu Agency. Retrieved 7 February 2021.
- ^ “Bolívia autoriza uso de vacinas Sputnik V e CoronaVac contra covid-19”. noticias.uol.com.br (in Portuguese). Retrieved 6 January 2021.
- ^ McGeever J, Fonseca P (17 January 2021). “Brazil clears emergency use of Sinovac, AstraZeneca vaccines, shots begin”. Reuters. Retrieved 17 January 2021.
- ^ Chanritheara, Torn. “Cambodia Approves AstraZeneca and Sinovac Vaccines for COVID-19 Emergency Use”. Cambodianess. Retrieved 12 February 2021.
- ^ “Chile aprueba el uso de emergencia de la vacuna china de Sinovac contra covid-19”. France 24. 20 January 2021. Retrieved 30 January 2021.
- ^ Aliyev J. “Colombia approves emergency use of CoronaVac vaccine”. Anadolu Agency. Retrieved 7 February 2021.
- ^ “Anticovid vaccines run out as Dominican Republic awaits arrival of more doses”. DominicanToday. Retrieved 10 March 2021.
- ^ “Ecuador signs agreement with Sinovac for 2 million COVID-19 vaccine: minister”. National Post. Retrieved 26 February 2021.
- ^ “Use of Sinovac vaccine authorised”. Government of Hong Kong. 18 February 2021. Retrieved 19 February 2021.
- ^ Soeriaatmadja W (11 January 2021). “Indonesia grants emergency use approval to Sinovac’s vaccine, local trials show 65% efficacy”. The Straits Times. Retrieved 11 January 2021.
- ^ “BPOM Grants Emergency Use Authorization for Sinovac Vaccine”. Tempo. 11 January 2021. Retrieved 11 January 2021.
- ^ Barrera, Adriana (11 February 2021). “Mexico approves China’s CanSino and Sinovac COVID-19 vaccines”. Reuters. Retrieved 11 February 2021.
- ^ “CoronaVac, vacuna de alta eficacia”. Ministerio de Salud Publica Y Bienestar Social.
- ^ “Philippines approves Sinovac’s COVID-19 vaccine for emergency use”. Reuters. 22 February 2021.
- ^ Thepgumpanat, Panarat (22 February 2021). “Thailand allows emergency use of Sinovac’s COVID-19 vaccine”. Reuters. Retrieved 23 February 2021.
- ^ “Tunisia approva vaccino cinese Sinovac” (in Italian). Agenzia Nazionale Stampa Associata (in Italian). 5 March 2021. Retrieved 7 March 2021.
- ^ “Turkey to begin COVID-19 vaccine jabs by this weekend”. Anadolu. 11 January 2021. Retrieved 11 January 2021.
- ^ Zinets, Natalia (9 March 2021). “Ukraine approves China’s Sinovac COVID-19 vaccine”. Reuters. Retrieved 10 March 2021.
- ^ “Covid-19: Zimbabwe authorises Sputnik V, Sinovac vaccines for emergency use”. news24.com. 9 March 2021.
- ^ “Regulation and Prequalification”. World Health Organization. Retrieved 12 March 2021.
- ^ Simoes E (30 September 2020). “Brazil’s Sao Paulo signs agreement with Sinovac for COVID vaccine doses”. Reuters. Archived from the original on 1 October 2020. Retrieved 1 October 2020.
- ^ Fonseca I (30 October 2020). “CoronaVac May Be Four Times More Costly Than Flu Vaccine”. The Rio Times. Archived from the original on 3 November 2020. Retrieved 30 October 2020.
- ^ “Em meio a críticas por atrasos, Pazuello diz que Brasil está preparado para iniciar vacinação em janeiro”. Folha de S.Paulo(in Portuguese). 6 January 2021. Retrieved 7 January 2021.
- ^ Rochabrun, Marcelo. “Brazil health ministry says plans to order 30 million more Coronavac doses | The Chronicle Herald”. http://www.thechronicleherald.ca. Retrieved 26 February 2021.
- ^ “Bolívia autoriza uso de vacinas Sputnik V e CoronaVac contra covid-19”. noticias.uol.com.br (in Portuguese). Retrieved 7 January 2021.
- ^ “Government meets with Sinovac for first COVID-19 vaccine clinical trial in Chile”. Government of Chile. 13 October 2020. Archived from the original on 17 October 2020. Retrieved 8 November 2020.
- ^ Presse, AFP-Agence France. “Chile Approves Chinese Coronavirus Vaccine”. barrons.com. Retrieved 21 January 2021.
- ^ “Fifth shipment with over two million Sinovac vaccines arrives to Chile”. Chile Reports. Retrieved 12 March 2021.
- ^ “Colombia extends health state of emergency, seeks more Sinovac vaccines”. Reuters. Retrieved 26 February 2021.
- ^ MENAFN. “Colombia declares emergency use of Sinovac vaccines”. menafn.com. Retrieved 4 February 2021.
- ^ “Ecuador signs agreement with Sinovac for 2 million COVID-19 vaccine: minister”. nationalpost. Retrieved 26 February 2021.
- ^ Jump up to:a b Valencia, Alexandra (7 March 2021). “Chile donates 40,000 doses of Sinovac vaccine to Ecuador and Paraguay”. Reuters. Retrieved 7 March 2021.
- ^ “CoronaVac, vacuna de alta eficacia”. Ministerio de Salud Publica Y Bienestar Social.
- ^ “Uruguay will receive first batches of Pfizer and Sinovac vaccines late February or early March: US$ 120 million investment”. MercoPress. Retrieved 24 January 2021.
- ^ “Albania gets 192,000 doses of Chinese Sinovac vaccine”. CNA. Retrieved 25 March 2021.
- ^ “Turkey signs 50 million dose COVID-19 vaccine deal, health minister says”. Reuters. 25 November 2020. Archived from the original on 1 December 2020. Retrieved 27 November 2020.
- ^ “Turkey grants emergency authorization to Sinovac’s CoronaVac: Anadolu”. Reuters. 13 January 2021. Retrieved 15 January 2021.
- ^ “Turkish president gets COVID-19 vaccine”. Anadolu Agency. 14 January 2021. Retrieved 20 January 2021.
- ^ SABAH, DAILY (12 March 2021). “Few virus infections reported among vaccinated people in Turkey”. Daily Sabah. Retrieved 12 March 2021.
- ^ “Ukraine signs up for China’s Sinovac vaccine, with doses expected soon”. Reuters. 30 December 2020. Retrieved 30 December 2020.
- ^ Zinets, Natalia (9 March 2021). “Ukraine approves China’s Sinovac COVID-19 vaccine”. Reuters. Retrieved 9 March 2021.
- ^ Aliyev, Jeyhun (19 January 2021). “Azerbaijan kicks off COVID-19 vaccination”. Anadolu Agency.
- ^ “Cambodian PM okays two more Covid-19 vaccines – Sinovac and AstraZeneca – for emergency use | The Star”. http://www.thestar.com.my. Retrieved 19 March 2021.
- ^ “Have no fear about shortage of vaccines, 1.5 million doses of Sinovac arriving on March 26”. Khmer Times. 19 March 2021. Retrieved 19 March 2021.
- ^ “Sinovac’s coronavirus vaccine candidate approved for emergency use in China – source”. Reuters. 29 August 2020. Archived from the original on 31 August 2020. Retrieved 30 August 2020.
- ^ “Government announces latest development of COVID-19 vaccine procurement” Archived 11 December 2020 at the Wayback Machine (Hong Kong Government Press Releases, 12 December 2020)
- ^ “Hong Kong kicks off COVID-19 vaccinations with Sinovac jab”. AP NEWS. 26 February 2021. Retrieved 7 March 2021.
- ^ “Indonesia books 50 million coronavirus vaccine doses from Sinovac”. Reuters. 21 August 2020. Archived from the original on 29 August 2020. Retrieved 21 August 2020.
- ^ “Sinovac vaccine has no critical side effects, BPOM says”. The Jakarta Post. Retrieved 21 December 2020.
- ^ Arkyasa, Mahinda (25 March 2021). “16 Million Sinovac Vaccines Material Arrives in Indonesia”. Tempo. Retrieved 25 March 2021.
- ^ “Malaysia’s NPRA Approves AstraZeneca, Sinovac Covid-19 Vaccines”. CodeBlue. 2 March 2021. Retrieved 2 March 2021.
- ^ Babulal, Veena (18 March 2021). “KJ gets first dose of Sinovac vaccine [NSTTV] | New Straits Times”. NST Online. Retrieved 19 March 2021.
- ^ “Duque says deal sealed for 25M doses of Sinovac COVID-19 vaccine”. GMA News Online. Retrieved 10 January 2021.
- ^ “Philippines receives COVID-19 vaccine after delays”. AP NEWS. 28 February 2021. Retrieved 28 February 2021.
- ^ Chen F (24 December 2020). “Brazil joins ranks of Chinese vaccine backers”. Asia Times Online. Retrieved 30 December2020.
- ^ “Singapore receives China’s Sinovac vaccine ahead of approval”. The Star. 25 February 2021. Retrieved 26 February2021.
- ^ “Thailand to get 2 million shots of China’s Sinovac”. Bangkok Post. Bangkok Post Public Company. Retrieved 4 January 2021.
- ^ “Thailand gives emergency use authorisation for Sinovac’s COVID-19 vaccine – official”. Reuters. 22 February 2021. Retrieved 23 February 2021.
- ^ Limited, Bangkok Post Public Company. “Thailand in talks to buy another 5m Sinovac shots”. Bangkok Post. Retrieved 20 March2021.
- ^ “Mexico approves China’s CanSino and Sinovac COVID-19 vaccines”. Reuters. 11 February 2021. Retrieved 11 February2021.
- ^ Jorgic, Drazen (10 March 2021). “Mexico leans on China after Biden rules out vaccines sharing in short term”. Reuters. Retrieved 10 March 2021.
- ^ Exteriores, Secretaría de Relaciones. “The Mexican Government receives 200,000 Sinovac COVID-19 vaccines”. gob.mx (in Spanish). Retrieved 7 March 2021.
- ^ “Lutte contre la Covid-19 : 203.000 doses de vaccins s dont 100.000 offertes par la Chine au Bénin”. Concentrées d’informations sur le Bénin et le monde à votre service depuis 2009(in French). 23 March 2021. Retrieved 25 March 2021.
- ^ Winning, Alexander. “South Africa’s drugs regulator to start assessing Sinovac COVID-19 vaccine”. U.S. Retrieved 12 March2021.
- ^ Nijini, Felix (18 March 2021). “Sinovac May Supply South Africa With 5 Million Vaccines: Report – BNN Bloomberg”. BNN. Retrieved 19 March 2021.
- ^ “Covid: Tunisia approva vaccino cinese Sinovac”. Agenzia Nazionale Stampa Associata (in Italian). 5 March 2021. Retrieved 7 March 2021.
- ^ Dzirutwe, MacDonald (10 March 2021). “Zimbabwe authorises Sputnik V, Sinovac coronavirus vaccines for emergency use”. Reuters. Retrieved 13 March 2021.
- ^ “China to donate Sinovac Vaccine to Fiji”. Fiji Broadcasting Corporation. Retrieved 17 March 2021.
- ^ Jump up to:a b Phillips, Tom (10 November 2020). “Jair Bolsonaro claims ‘victory’ after suspension of Chinese vaccine trial”. The Guardian. Retrieved 18 January 2021.
- ^ Baptista, Eduardo (11 December 2020). “China-made coronavirus vaccine at heart of political showdown in Brazil”. South China Morning Post. Retrieved 18 January 2021.
- ^ Carvalho, Daniel (14 January 2021). “‘Is 50% Good?’, Asks Bolsonaro, Mocking Coronavac’s Effectiveness”. Folha de S.Paulo. Retrieved 18 January 2021.
- ^ Pearson, Samantha; Magalhaes, Luciana (10 November 2020). “Brazil’s Medical Experts Worry Politics Is Hampering Covid-19 Vaccine Progress”. The Wall Street Journal. Retrieved 18 January 2021.
- ^ “Covid: 70% dos brasileiros não fazem questão de escolher vacina” [Covid: 70% of Brazilians do not make a point of choosing vaccine]. R7.com (in Portuguese). 3 March 2021. Retrieved 9 March2021.
- ^ Fonseca P. “Brazil institute says CoronaVac efficacy above 50%, but delays full results”. Reuters. Retrieved 25 December 2020.
- ^ Hong, Jinshan (12 January 2021). “How Effective Is China’s Sinovac Vaccine? Data Confuse Experts”. Bloomberg News. Retrieved 12 January 2021.
External links
- Clinical Research Protocol for CoronaVac Phase III Trials in Brazil
- Clinical Research Protocol for CoronaVac Phase III Trials in Chile
- “How the Sinovac Covid-19 Vaccine Works”. The New York Times.
| Vaccine description | |
|---|---|
| Target | SARS-CoV-2 |
| Vaccine type | Inactivated |
| Clinical data | |
| Routes of administration | Intramuscular injection |
| ATC code | None |
| Legal status | |
| Legal status | Emergency authorization for use in China, Indonesia, Brazil and Turkey |
| Identifiers | |
| DrugBank | DB15806 |
| Part of a series on the |
| COVID-19 pandemic |
|---|
| SARS-CoV-2 (virus)COVID-19 (disease) |
| showTimeline |
| showLocations |
| showInternational response |
| showMedical response |
| showImpact |
| COVID-19 Portal |
| vte |
Sinovac Biotech Ltd. (Chinese: 北京科兴生物制品有限公司, Nasdaq: SVA) is a Chinese biopharmaceutical company that focuses on the research, development, manufacture and commercialization of vaccines that protect against human infectious diseases. The company is based in Haidian District, Beijing.[2] The company is listed on the NASDAQ but the exchange halted Sinovac’s trading in February 2019 due to a proxy fight.[3][4]
Vaccines
Sinovac’s commercialized vaccines include Healive (hepatitis A), Bilive (combined hepatitis A and B), Anflu (influenza), Panflu (H5N1) and PANFLU.1 (H1N1). Sinovac is currently developing a Universal Pandemic Influenza vaccine and a Japanese encephalitis vaccine.[5][better source needed]
Sinovac is also developing vaccines for enterovirus 71 and human rabies. Its wholly owned subsidiary, Tangshan Yian, is conducting field trials for independently developed inactivated animal rabies vaccines.[citation needed]
COVID-19 vaccine development
Main article: CoronaVac
CoronaVac is an inactivated virus COVID-19 vaccine developed by Sinovac.[6] It has been in Phase III clinical trials in Brazil,[7] Chile,[8] Indonesia,[9] Malaysia,[10] Philippines,[11] and Turkey.[12]
It relies on traditional technology similar to BBIBP-CorV and BBV152, other inactivated-virus COVID-19 vaccines in Phase III trials.[13] CoronaVac does not need to be frozen, and both the vaccine and raw material for formulating the new doses could be transported and refrigerated at 2–8 °C (36–46 °F), temperatures at which flu vaccines are kept.[14]
Brazil announced results on January 13, 2021 showing 50.4% effective at preventing symptomatic infections, 78% effective in preventing mild cases needing treatment, and 100% effective in preventing severe cases.[15] Final Phase III results from Turkey announced on 3 March 2021 showed an efficacy of 83.5%.[16] Interim results in Indonesia were announced on 11 January 2021 with an efficacy of 65.3%.[17]
CoronaVac is being used in vaccination campaigns by certain countries in Asia,[18][19][20] South America,[21][22] and Europe.[23] In March, a Sinovac spokesman told Reuters production capacity for CoronaVac could reach 2 billion doses a year by June 2021.[24] As of 27 February 36 million doses had been administered in total.[25]
See also
References
- ^ “China’s Vaccine Front-Runner Aims to Beat Covid the Old-Fashioned Way”. Bloomberg. 24 August 2020.
- ^ “Home (English)”. Sinovac. Retrieved 2021-03-06.
Add: No. 39 Shangdi Xi Road, Haidian District, Beijing, P.R.C. 100085
– Chinese address: “地址:中国· 北京 海淀区上地西路39号北大生物城(100085)” - ^ Dou, Eva (December 4, 2020). “As China nears a coronavirus vaccine, bribery cloud hangs over drugmaker Sinovac”. The Washington Post. ISSN 0190-8286. Archived from the original on December 4, 2020. Retrieved 2020-12-06.
- ^ Levine, Matt (May 22, 2020). “A Vaccine With a Poison Pill”. Bloomberg News. Archived from the original on June 21, 2020. Retrieved December 6, 2020.
- ^ Google Finance, url=https://www.google.com/finance?q=Sinovac
- ^ Nidhi Parekh (22 July 2020). “CoronaVac: A COVID-19 Vaccine Made From Inactivated SARS-CoV-2 Virus”. Retrieved 25 July2020.
- ^ “New coronavirus vaccine trials start in Brazil”. AP News. 21 July 2020. Retrieved 2020-10-07.
- ^ “Chile initiates clinical study for COVID-19 vaccine”. Chile Reports. 4 August 2020. Retrieved 2020-10-07.
- ^ “248 volunteers have received Sinovac vaccine injections in Bandung”. Antara News. 30 August 2020. Retrieved 2020-10-07.
- ^ “Malaysia Receives China’s Sinovac Vaccine For Regulatory Testing”. Bloomberg.com. 2021-02-27. Retrieved 2021-03-02.
- ^ “DOH eyes 5 hospitals for Sinovac vaccine Phase 3 clinical trial”. PTV News. 16 September 2020. Retrieved 2020-10-07.
- ^ “Turkey begins phase three trials of Chinese Covid-19 vaccine”. TRT World News. 1 September 2020. Retrieved 2020-10-07.
- ^ Zimmer, Carl; Corum, Jonathan; Wee, Sui-Lee. “Coronavirus Vaccine Tracker”. The New York Times. ISSN 0362-4331. Retrieved 2021-02-12.
- ^ “CoronaVac: Doses will come from China on nine flights and can…” AlKhaleej Today (in Arabic). 2020-11-01. Retrieved 2021-02-12.
- ^ “Sinovac: Brazil results show Chinese vaccine 50.4% effective”. BBC News. 2021-01-13. Retrieved 2021-02-12.
- ^ AGENCIES, DAILY SABAH WITH (25 December 2020). “Turkey set to receive ‘effective’ COVID-19 vaccine amid calls for inoculation”. Daily Sabah. Retrieved 12 February 2021.
- ^ hermesauto (11 January 2021). “Indonesia grants emergency use approval to Sinovac’s vaccine, local trials show 65% efficacy”. The Straits Times. Retrieved 12 February 2021.
- ^ TARIGAN, EDNA; MILKO, VICTORIA (13 January 2021). “Indonesia starts mass COVID vaccinations over vast territory”. Associated Press. Retrieved 15 January 2021.
- ^ Aliyev, Jeyhun (19 January 2021). “Azerbaijan kicks off COVID-19 vaccination”. Anadolu Agency.
- ^ “China approves Sinovac vaccines for general public use”. South China Morning Post. 6 February 2021. Retrieved 6 February2021.
- ^ Fonseca, Jamie McGeever, Pedro (17 January 2021). “Brazil clears emergency use of Sinovac, AstraZeneca vaccines, shots begin”. Reuters. Retrieved 17 January 2021.
- ^ Miranda, Natalia A. Ramos (28 January 2021). “Chile receives two million-dose first delivery of Sinovac COVID-19 vaccine”. Reuters. Retrieved 30 January 2021.
- ^ “Turkey aims to vaccinate 60 percent of population: Minister – Turkey News”. Hürriyet Daily News. Retrieved 12 February 2021.
- ^ Liu, Roxanne (2021-03-03). “Sinovac eyes two billion doses in annual capacity of virus vaccine by June”. Reuters. Retrieved 2021-03-03.
- ^ “Malaysia receives first batch of Sinovac Covid-19 vaccine today”. Bernama. 27 February 2021. Retrieved 27 February 2021– via The Malay Mail.
External links
- Official website
- Business data for Sinovac Biotech:
| Type | Public |
|---|---|
| Traded as | Nasdaq: SVA (American Depository Receipts) |
| Founded | 1999; 22 years ago |
| Founder | Yin Weidong[1] |
| Headquarters | Beijing,China |
| Website | http://www.sinovac.com/ |
| Sinovac Biotech | |
|---|---|
| Simplified Chinese | 北京科兴生物制品有限公司 |
| Traditional Chinese | 北京科興生物製品有限公司 |
| hideTranscriptionsStandard MandarinHanyu PinyinBěijīng Kē Xìng Shēngwù Zhìpǐn Yǒuxiàn Gōngsī |
/////////Sinovac COVID-19 vaccine, CoronaVac, corona virus, covid 19, vaccine, china, Sinovac Biotech, PiCoVacc
#Sinovac COVID-19 vaccine, #CoronaVac, #corona virus, #covid 19, #vaccine, #china, #Sinovac Biotech, #PiCoVacc