

Lenalidomide hydrate
レナリドミド水和物
An immunomodulator.
CC-5013 hemihydrate
2,6-Piperidinedione, 3-(4-amino-1,3-dihydro-1-oxo-2H-isoindol-2-yl)-, hydrate (2:1)
(+/-)-2,6-Piperidinedione, 3-(4-amino-1,3-dihydro-1-oxo-2H-isoindol-2-yl)-, hydrate (2:1)
| Formula | (C13H13N3O3)2. H2O |
|---|---|
| CAS | 847871-99-2 |
| Mol weight | 536.5365 |
EMA APPROVED 2021/2/11, Lenalidomide KRKA
Research Code:CDC-501; CC-5013
Trade Name:Revlimid®
MOA:Angiogenesis inhibitor
Indication:Myelodysplastic syndrome (MDS); Mantle cell lymphoma (MCL); Multiple myeloma (MM)
Status:Approved
Company:Celgene (Originator)
Sales:$5,801.1 Million (Y2015); 
$4,980 Million (Y2014);;
$4280 Million (Y2013);;
$3766.6 Million (Y2012);;
$3208.2 Million (Y2011);ATC Code:L04AX04
| Approval Date | Approval Type | Trade Name | Indication | Dosage Form | Strength | Company | Review Classification |
|---|---|---|---|---|---|---|---|
| 2005-12-27 | Marketing approval | Revlimid | Multiple myeloma (MM),Myelodysplastic syndrome (MDS),Mantle cell lymphoma (MCL) | Capsule | 2.5 mg/5 mg/10 mg/15 mg/20 mg/25 mg | Celgene | Priority; Orphan |
| Approval Date | Approval Type | Trade Name | Indication | Dosage Form | Strength | Company | Review Classification |
|---|---|---|---|---|---|---|---|
| 2007-06-14 | Marketing approval | Revlimid | Multiple myeloma (MM),Myelodysplastic syndrome (MDS) | Capsule | 2.5 mg/5 mg/7.5 mg/10 mg/15 mg/20 mg/25 mg | Celgene | Orphan |
| Approval Date | Approval Type | Trade Name | Indication | Dosage Form | Strength | Company | Review Classification |
|---|---|---|---|---|---|---|---|
| 2010-08-20 | New indication | Revlimid | Myelodysplastic syndrome (MDS) | Capsule | 5 mg | Celgene | |
| 2010-06-25 | Marketing approval | Revlimid | Multiple myeloma (MM) | Capsule | 5 mg | Celgene |
| Approval Date | Approval Type | Trade Name | Indication | Dosage Form | Strength | Company | Review Classification |
|---|---|---|---|---|---|---|---|
| 2013-01-23 | Marketing approval | 瑞复美/Revlimid | Multiple myeloma (MM) | Capsule | 5 mg | Celgene | |
| 2013-01-23 | Marketing approval | 瑞复美/Revlimid | Multiple myeloma (MM) | Capsule | 10 mg | Celgene | |
| 2013-01-23 | Marketing approval | 瑞复美/Revlimid | Multiple myeloma (MM) | Capsule | 15 mg | Celgene | |
| 2013-01-23 | Marketing approval | 瑞复美/Revlimid | Multiple myeloma (MM) | Capsule | 25 mg | Celgene |
| Molecular Weight | 259.26 |
| Formula | C13H13N3O3 |
| CAS No. | 191732-72-6 (Lenalidomide); |
| Chemical Name | 3(4-amino-1-oxo 1,3-dihydro-2H-isoindol-2-yl) piperidine-2,6-dione |
Lenalidomide was first approved by the U.S. Food and Drug Administration (FDA) on Dec 27, 2005, then approved by European Medicine Agency (EMA) on June 14, 2007, and approved by Pharmaceuticals and Medical Devices Agency of Japan (PMDA) on June 25, 2010. It was developed and marketed as Revlimid® by Celgene.
Lenalidomide is an analogue of thalidomide with immunomodulatory, antiangiogenic, and antineoplastic properties. In multiple myeloma cells, the combination of lenalidomide and dexamethasone synergizes the inhibition of cell proliferation and the induction of apoptosis. Revlimid® is indicated for the treatment of multiple myeloma (MM), in combination with dexamethasone, in patients who have received at least one prior therapy, transfusion-dependent anemia due to low-or intermediate-1-risk myelodysplastic syndromes (MDS) associated with a deletion 5q abnormality with or without additional cytogenetic abnormalities and mantle cell lymphoma (MCL) whose disease has relapsed or progressed after two prior therapies, one of which included bortezomib.
Revlimid® is available as capsule for oral use, containing 2.5, 5, 10, 15, 20 or 25 mg of free Lenalidomide. The recommended dose is 25 mg once daily for multiple myeloma (MM), in combination with 40 mg dexamethasone once daily, 10 mg once daily for myelodysplastic syndromes (MDS) and 25 mg once daily for mantle cell lymphoma (MCL).
Lenalidomide, sold under the trade name Revlimid among others, is a medication used to treat multiple myeloma (MM) and myelodysplastic syndromes (MDS).[2] For MM it is used after at least one other treatment and generally together with dexamethasone.[2] It is taken by mouth.[2]
Common side effects include diarrhea, itchiness, joint pain, fever, headache, and trouble sleeping.[2] Severe side effects may include low blood platelets, low white blood cells, and blood clots.[2] Use during pregnancy may harm the baby.[2] The dose may need to be adjusted in people with kidney problems.[2] It has a chemical structure similar to thalidomide but has a different mechanism of action.[3][2] How it works is not entirely clear as of 2019.[2]
Lenalidomide was approved for medical use in the United States in 2005.[2] It is on the World Health Organization’s List of Essential Medicines.[4]
Medical uses
Multiple myeloma
Lenalidomide is used to treat multiple myeloma.[5] It is a more potent molecular analog of thalidomide, which inhibits tumor angiogenesis, tumor-secreted cytokines, and tumor proliferation through induction of apoptosis.[6][7][8]
Lenalidomide is effective at inducing a complete or “very good partial” response and improves progression-free survival. Adverse events more common in people receiving lenalidomide for myeloma include neutropenia, deep vein thrombosis, infections, and an increased risk of other hematological malignancies.[9] The risk of second primary hematological malignancies does not outweigh the benefit of using lenalidomide in relapsed or refractory multiple myeloma.[10] It may be more difficult to mobilize stem cells for autograft in people who have received lenalidomide.[6]
In 2006, lenalidomide received U.S. Food and Drug Administration (FDA) clearance for use in combination with dexamethasone in people with multiple myeloma who have received at least one prior therapy.[11] In 2017, the FDA approved lenalidomide as standalone maintenance therapy (without dexamethasone) for people with multiple myeloma following autologous stem cell transplant.[12]
In 2009, The National Institute for Health and Clinical Excellence issued a final appraisal determination approving lenalidomide in combination with dexamethasone as an option to treat people with multiple myeloma who have received two or more prior therapies in England and Wales.[13]
The use of lenalidomide combined with other drugs was evaluated. It was seen that the drug combinations of lenalidomide plus dexamethasone and continuous bortezomib plus lenalidomide plus dexamethasone probably result in an increase of the overall survival.[14]
Myelodysplastic syndromes
Lenalidomide was approved by the FDA on 27 December 2005 for patients with low- or intermediate-1-risk myelodysplastic syndromes who have chromosome 5q deletion syndrome (5q- syndrome) with or without additional cytogenetic abnormalities.[15][16][17] It was approved on 17 June 2013 by the European Medicines Agency for use in patients with low- or intermediate-1-risk myelodysplastic syndromes who have 5q- deletion syndrome but no other cytogenetic abnormalities and are dependent on red blood cell transfusions, for whom other treatment options have been found to be insufficient or inadequate.[18]
Mantle cell lymphoma
Lenalidomide is approved by FDA as a specialty drug requiring a specialty pharmacy distribution for mantle cell lymphoma in patients whose disease has relapsed or progressed after at least two prior therapies, one of which must have included the medicine bortezomib.[3]
Amyloidosis
Although not specifically approved by the FDA for use in treating amyloidosis, Lenalidomide is widely used in the treatment of that condition, often in combination with dexamethasone. [19]
Adverse effects
In addition to embryo-fetal toxicity, lenalidomide carries black box warnings for hematologic toxicity (including neutropenia and thrombocytopenia) and thromboembolism.[3] Serious potential side effects include thrombosis, pulmonary embolus, hepatotoxicity, and bone marrow toxicity resulting in neutropenia and thrombocytopenia. Myelosuppression is the major dose-limiting toxicity, which is not the case with thalidomide.[20]
Lenalidomide may be associated with such adverse effects as second primary malignancy, severe cutaneous reactions, hypersensitivity reactions, tumor lysis syndrome, tumor flare reaction, hypothyroidism, and hyperthyroidism.[3]
Teratogenicity
Lenalidomide is related to thalidomide, which is known to be teratogenic. Tests in monkeys suggest that lenalidomide is likewise teratogenic.[21] It cannot be prescribed for women who are pregnant or who may become pregnant during therapy.[1] For this reason, the drug is only available in the United States through a restricted distribution system in conjunction with a risk evaluation and mitigation strategy. Females who may become pregnant must use at least two forms of reliable contraception during treatment and for at least four weeks after discontinuing treatment with lenalidomide.[3][22]
Venous thromboembolism
Lenalidomide, like its parent compound thalidomide, may cause venous thromboembolism (VTE), a potentially serious complication with their use. High rates of VTE have been found in patients with multiple myeloma who received thalidomide or lenalidomide in conjunction with dexamethasone, melphalan, or doxorubicin.[23]
Stevens-Johnson syndrome
In March 2008, the U.S. Food and Drug Administration (FDA) included lenalidomide on a list of twenty prescription drugs under investigation for potential safety problems. The drug was investigated for possibly increasing the risk of developing Stevens–Johnson syndrome, a life-threatening skin condition.[24]
FDA ongoing safety review
In 2011, the FDA initiated an ongoing review of clinical trials that found an increased risk of developing cancers such as acute myelogenous leukemia and B-cell lymphoma,[25] though it did not advise patients to discontinue treatment with lenalidomide.[26]
Mechanism of action
Lenalidomide has been used to successfully treat both inflammatory disorders and cancers in the past ten years.[when?] There are multiple mechanisms of action, and they can be simplified by organizing them as mechanisms of action in vitro and in vivo.[27] In vitro, lenalidomide has three main activities: direct anti-tumor effect, inhibition of angiogenesis, and immunomodulation. In vivo, lenalidomide induces tumor cell apoptosis directly and indirectly by inhibition of bone marrow stromal cell support, by anti-angiogenic and anti-osteoclastogenic effects, and by immunomodulatory activity. Lenalidomide has a broad range of activities that can be exploited to treat many hematologic and solid cancers.
On a molecular level, lenalidomide has been shown to interact with the ubiquitin E3 ligase cereblon[28] and target this enzyme to degrade the Ikaros transcription factors IKZF1 and IKZF3.[29] This mechanism was unexpected as it suggests that the major action of lenalidomide is to re-target the activity of an enzyme rather than block the activity of an enzyme or signaling process, and thereby represents a novel mode of drug action. A more specific implication of this mechanism is that the teratogenic and anti-neoplastic properties of lenalidomide, and perhaps other thalidomide derivatives, could be disassociated.
History
See also: Development of analogs of thalidomide
Lenalidomide was approved for medical use in the United States in 2005.[2]
Society and culture
Economics
Lenalidomide costs US$163,381 per year for the average person in the United States as of 2012.[25] Lenalidomide made almost $9.7bn for Celgene in 2018.[30]
In 2013, the UK National Institute for Health and Care Excellence (NICE) rejected lenalidomide for “use in the treatment of people with a specific type of the bone marrow disorder myelodysplastic syndrome (MDS)” in England and Scotland, arguing that Celgene “did not provide enough evidence to justify the GB£3,780 per month (US$5,746.73) price-tag of lenalidomide for use in the treatment of people with a specific type of the bone marrow disorder myelodysplastic syndrome (MDS)”.[31]
Research
Lenalidomide is undergoing clinical trial as a treatment for Hodgkin’s lymphoma,[32] as well as non-Hodgkin’s lymphoma, chronic lymphocytic leukemia and solid tumor cancers, such as carcinoma of the pancreas.[33] One Phase III clinical trial being conducted by Celgene in elderly patients with B-cell chronic lymphocytic leukemia was halted in July 2013, when a disproportionate number of cancer deaths were observed during treatment with lenalidomide versus patients treated with chlorambucil.[34]
1. WO9803502A1 / US2002173658A1.
2. Bioorg. Med. Chem. Lett. 1999, 9, 1625-1630.Route 2
Reference:
1. WO2010139266A1 / US2012077982A1.Route 3
Reference:
1. CN103497175A.Route 4
Reference:
1. WO2010139266A1 / US2012077982A1.Route 5
Reference:
1. CN103554082A.
Clip

SYN

SCALABLE AND GREEN PROCESS FOR THE SYNTHESIS OF ANTICANCER DRUG LENALIDOMIDE
Yuri Ponomaryov, Valeria Krasikova, Anton Lebedev, Dmitri Chernyak, Larisa Varacheva, Alexandr Chernobroviy

Abstract
A new process for the synthesis of anticancer drug lenalidomide was developed, using platinum group metal-free and efficient reduction of nitro group with the iron powder and ammonium chloride. It was found that the bromination of the key raw material, methyl 2-methyl-3-nitrobenzoate, could be carried out in chlorine-free solvent methyl acetate without forming significant amounts of hazardous by-products. We also have compared the known synthetic methods for cyclization of methyl 2-(bromomethyl)-3-nitrobenzoate and 3-aminopiperidinedione to form lenalidomide nitro precursor.
How to Cite
Ponomaryov, Y.; Krasikova, V.; Lebedev, A.; Chernyak, D.; Varacheva, L.; Chernobroviy, A. Chem. Heterocycl. Compd. 2015, 51, 133. [Khim. Geterotsikl. Soedin. 2015, 51, 133.]
For this article in the English edition see DOI 10.1007/s10593-015-1670-0
SYN
https://link.springer.com/article/10.1007/s10593-015-1670-0

A new process for the synthesis of anticancer drug lenalidomide was developed, using platinum group metal-free and efficient reduction of nitro group with the iron powder and ammonium chloride. It was found that the bromination of the key raw material, methyl 2-methyl-3-nitrobenzoate, could be carried out in chlorine-free solvent methyl acetate without forming significant amounts of hazardous by-products. We also have compared the known synthetic methods for cyclization of methyl 2-(bromomethyl)-3-nitrobenzoate and 3-aminopiperidinedione to form lenalidomide nitro precursor.
SYN

SYN
EP 0925294; US 5635517; WO 9803502
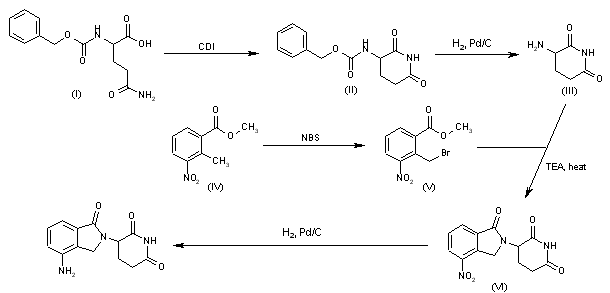
Cyclization of N-(benzyloxycarbonyl)glutamine (I) by means of CDI in refluxing THF gives 3-(benzyloxycarbonylamino)piperidine-2,6-dione (II), which is deprotected with H2 over Pd/C in ethyl acetate/4N HCl to yield 3-aminopiperidine-2,6-dione hydrochloride (III). Bromination of 2-methyl-3-nitrobenzoic acid methyl ester (IV) with NBS in CCl4 provides 2-(bromomethyl)-3-nitrobenzoic acid methyl ester (V), which is cyclized with the aminopiperidine (III) by means of triethylamine in hot DMF to afford 3-(4-nitro-1-oxoisoindolin-2-yl)piperidine-2,6-dione (VI). Finally, the nitro group of compound (VI) is reduced with H2 over Pd/C in methanol (1, 2).
SYN
Bioorg Med Chem Lett 1999,9(11),1625
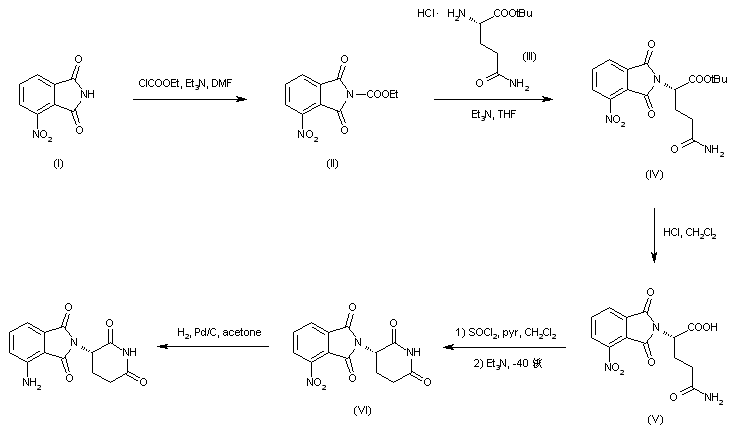
Treatment of 3-nitrophthalimide (I) with ethyl chloroformate and triethylamine produced 3-nitro-N-(ethoxycarbonyl)phthalimide (II), which was condensed with L-glutamine tert-butyl ester hydrochloride (III) to afford the phthaloyl glutamine derivative (IV). Acidic cleavage of the tert-butyl ester of (IV) provided the corresponding carboxylic acid (V). This was cyclized to the required glutarimide (VI) upon treatment with thionyl chloride and then with triethylamine. The nitro group of (VI) was finally reduced to amine by hydrogenation over Pd/C.
Lenalidomide
- Synonyms:CC-5013, CDC 501
- ATC:L04AX04
- MW:259.27 g/mol
- CAS-RN:191732-72-6
- InChI Key:GOTYRUGSSMKFNF-JTQLQIEISA-N
- InChI:InChI=1S/C13H13N3O3/c14-9-3-1-2-7-8(9)6-16(13(7)19)10-4-5-11(17)15-12(10)18/h1-3,10H,4-6,14H2,(H,15,17,18)/t10-/m0/s1
Synthesis
References
- ^ Jump up to:a b c “Lenalidomide (Revlimid) Use During Pregnancy”. Drugs.com. 13 March 2020. Retrieved 13 August 2020.
- ^ Jump up to:a b c d e f g h i j k “Lenalidomide Monograph for Professionals”. Drugs.com. Retrieved 27 October 2019.
- ^ Jump up to:a b c d e “DailyMed – Revlimid- lenalidomide capsule”. dailymed.nlm.nih.gov. Retrieved 27 October 2019.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Armoiry X, Aulagner G, Facon T (June 2008). “Lenalidomide in the treatment of multiple myeloma: a review”. Journal of Clinical Pharmacy and Therapeutics. 33 (3): 219–26. doi:10.1111/j.1365-2710.2008.00920.x. PMID 18452408. S2CID 1228171.
- ^ Jump up to:a b Li S, Gill N, Lentzsch S (November 2010). “Recent advances of IMiDs in cancer therapy”. Current Opinion in Oncology. 22 (6): 579–85. doi:10.1097/CCO.0b013e32833d752c. PMID 20689431. S2CID 205547603.
- ^ Tageja N (March 2011). “Lenalidomide – current understanding of mechanistic properties”. Anti-Cancer Agents in Medicinal Chemistry. 11 (3): 315–26. doi:10.2174/187152011795347487. PMID 21426296.
- ^ Kotla V, Goel S, Nischal S, Heuck C, Vivek K, Das B, Verma A (August 2009). “Mechanism of action of lenalidomide in hematological malignancies”. Journal of Hematology & Oncology. 2: 36. doi:10.1186/1756-8722-2-36. PMC 2736171. PMID 19674465.
- ^ Yang B, Yu RL, Chi XH, Lu XC (2013). “Lenalidomide treatment for multiple myeloma: systematic review and meta-analysis of randomized controlled trials”. PLOS ONE. 8 (5): e64354. Bibcode:2013PLoSO…864354Y. doi:10.1371/journal.pone.0064354. PMC 3653900. PMID 23691202.
- ^ Dimopoulos MA, Richardson PG, Brandenburg N, Yu Z, Weber DM, Niesvizky R, Morgan GJ (March 2012). “A review of second primary malignancy in patients with relapsed or refractory multiple myeloma treated with lenalidomide”. Blood. 119 (12): 2764–7. doi:10.1182/blood-2011-08-373514. PMID 22323483.
- ^ “FDA approves lenalidomide oral capsules (Revlimid) for use in combination with dexamethasone in patients with multiple myeloma”. Food and Drug Administration (FDA). 29 June 2006. Retrieved 15 October 2015.[dead link]
- ^ “Lenalidomide (Revlimid)”. Food and Drug Administration(FDA). 22 February 2017.
- ^ “REVLIMID Receives Positive Final Appraisal Determination from National Institute for Health and Clinical Excellence (NICE) for Use in the National Health Service (NHS) in England and Wales”. Reuters. 23 April 2009.
- ^ Piechotta V, Jakob T, Langer P, Monsef I, Scheid C, Estcourt LJ, et al. (Cochrane Haematology Group) (November 2019). “Multiple drug combinations of bortezomib, lenalidomide, and thalidomide for first-line treatment in adults with transplant-ineligible multiple myeloma: a network meta-analysis”. The Cochrane Database of Systematic Reviews. 2019 (11). doi:10.1002/14651858.CD013487. PMC 6876545. PMID 31765002.
- ^ List A, Kurtin S, Roe DJ, Buresh A, Mahadevan D, Fuchs D, et al. (February 2005). “Efficacy of lenalidomide in myelodysplastic syndromes”. The New England Journal of Medicine. 352 (6): 549–57. doi:10.1056/NEJMoa041668. PMID 15703420.
- ^ List AF (August 2005). “Emerging data on IMiDs in the treatment of myelodysplastic syndromes (MDS)”. Seminars in Oncology. 32 (4 Suppl 5): S31-5. doi:10.1053/j.seminoncol.2005.06.020. PMID 16085015.
- ^ List A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E, et al. (October 2006). “Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion”. The New England Journal of Medicine. 355 (14): 1456–65. doi:10.1056/NEJMoa061292. PMID 17021321.
- ^ “Revlimid Approved In Europe For Use In Myelodysplastic Syndromes”. The MDS Beacon. Retrieved 17 June 2013.
- ^ “Revlimid and Amyloidosis AL” (PDF). MyelomaUK. Retrieved 3 October 2020.
- ^ Rao KV (September 2007). “Lenalidomide in the treatment of multiple myeloma”. American Journal of Health-System Pharmacy. 64 (17): 1799–807. doi:10.2146/ajhp070029. PMID 17724360.
- ^ “Revlimid Summary of Product Characteristics. Annex I” (PDF). European Medicines Agency. 2012. p. 6.
- ^ Ness, Stacey (13 March 2014). “New Specialty Drugs”. Pharmacy Times. Retrieved 5 November 2015.
- ^ Bennett CL, Angelotta C, Yarnold PR, Evens AM, Zonder JA, Raisch DW, Richardson P (December 2006). “Thalidomide- and lenalidomide-associated thromboembolism among patients with cancer”. JAMA. 296 (21): 2558–60. doi:10.1001/jama.296.21.2558-c. PMID 17148721.
- ^ “Potential Signals of Serious Risks/New Safety Information Identified from the Adverse Event Reporting System (AERS) between January – March 2008”. Food and Drug Administration(FDA). March 2008. Archived from the original on 19 April 2014. Retrieved 16 December 2019.
- ^ Jump up to:a b Badros AZ (May 2012). “Lenalidomide in myeloma–a high-maintenance friend”. The New England Journal of Medicine. 366(19): 1836–8. doi:10.1056/NEJMe1202819. PMID 22571206.
- ^ “FDA Drug Safety Communication: Ongoing safety review of Revlimid (lenalidomide) and possible increased risk of developing new malignancies”. Food and Drug Administration (FDA). April 2011.
- ^ Vallet S, Palumbo A, Raje N, Boccadoro M, Anderson KC (July 2008). “Thalidomide and lenalidomide: Mechanism-based potential drug combinations”. Leukemia & Lymphoma. 49 (7): 1238–45. doi:10.1080/10428190802005191. PMID 18452080. S2CID 43350339.
- ^ Zhu YX, Braggio E, Shi CX, Bruins LA, Schmidt JE, Van Wier S, et al. (November 2011). “Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide”. Blood. 118 (18): 4771–9. doi:10.1182/blood-2011-05-356063. PMC 3208291. PMID 21860026.
- ^ Stewart AK (January 2014). “Medicine. How thalidomide works against cancer”. Science. 343 (6168): 256–7. doi:10.1126/science.1249543. PMC 4084783. PMID 24436409.
- ^ “Top 10 Best-Selling Cancer Drugs of 2018”. Genetic Engineering and Biotechnology News. 22 April 2019. Retrieved 25 April 2019.
- ^ “Revlimid faces NICE rejection for use in rare blood cancer Watchdog’s draft guidance does not recommend Celgene’s drug for NHS use in England and Wales”. Pharma News. 11 July 2013. Retrieved 5 November 2015.
- ^ “Phase II Study of Lenalidomide for the Treatment of Relapsed or Refractory Hodgkin’s Lymphoma”. ClinicalTrials.gov. US National Institutes of Health. February 2009.
- ^ “276 current clinical trials world-wide, both recruiting and fully enrolled, as of 27 February 2009”. ClinicalTrials.gov. US National Institutes of Health. February 2009.
- ^ “Celgene Discontinues Phase 3 Revlimid Study after ‘Imbalance’ of Deaths”. Nasdaq. 18 July 2013.
External links[edit]
- “Lenalidomide”. Drug Information Portal. U.S. National Library of Medicine.
//////////Lenalidomide hydrate, Lenalidomide KRKA, EU 2021, APPROVALS 2021, レナリドミド水和物 , CC-5013 hemihydrate,
#Lenalidomide hydrate, #Lenalidomide KRKA, #EU 2021, #APPROVALS 2021, #レナリドミド水和物 , #CC-5013 hemihydrate,
O.Nc1cccc2C(=O)N(Cc12)C3CCC(=O)NC3=O.Nc4cccc5C(=O)N(Cc45)C6CCC(=O)NC6=O