Pyridostigmine
- Molecular FormulaC9H13N2O2
- Average mass181.211 Da
155-97-5[RN]3-[(Dimethylcarbamoyl)oxy]-1-methylpyridinium
3-Dimethylcarbamoyloxy-1-methyl-pyridinium5-21-02-00078 (Beilstein Handbook Reference)[Beilstein]
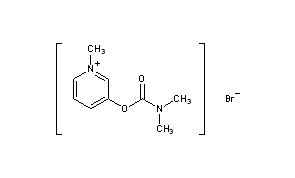
Pyridostigmine BromideCAS Registry Number: 101-26-8CAS Name: 3-[[(Dimethylamino)carbonyl]oxy]-1-methylpyridinium bromideAdditional Names: 3-hydroxy-1-methylpyridinium bromide dimethylcarbamate; 1-methyl-3-hydroxypyridinium bromide dimethylcarbamate; 3-(dimethylcarbamyloxy)-1-methylpyridinium bromideManufacturers’ Codes: Ro-1-5130Trademarks: Kalymin (Temmler); Mestinon (Roche); Regonol (Organon)Molecular Formula: C9H13BrN2O2Molecular Weight: 261.12Percent Composition: C 41.40%, H 5.02%, Br 30.60%, N 10.73%, O 12.25%Literature References: Reversible inhibitor of acetylcholinesterase.
Prepn: Urban, US2572579 (1951 to Hoffmann-La Roche). Mechanism of protective effect in soman poisoning: X. Deyi et al.,Fundam. Appl. Toxicol.1, 217 (1981). Evaluation of effect on neuromuscular function: M. Glikson et al.,ibid.16, 288 (1991). Evaluation of side effects profile under desert conditions: J. E. Cook et al.,Mil. Med.157, 250 (1992). Review of prophylactic effect in nerve agent poisoning: R. M. Dawson, J. Appl. Toxicol.14, 317 (1994).Properties: Shiny, hygroscopic crystals from abs ethanol, mp 152-154°. Freely sol in water, alcohol. Practically insol in ether, acetone, benzene. Aq solns may be sterilized by autoclaving with steam.Melting point: mp 152-154°Therap-Cat: Cholinergic; in treatment of myasthenia gravis. Pre-exposure antidote to chemical warfare agents.Keywords: Cholinergic.
Pyridostigmine is a medication used to treat myasthenia gravis.[1] It is also used together with atropine to end the effects of neuromuscular blocking medication of the non-depolarizing type.[2] It is typically given by mouth but can also be used by injection.[2] The effects generally begin within 45 minutes and last up to 6 hours.[2]
Common side effects include nausea, diarrhea, frequent urination, and abdominal pain.[2] More severe side effects include low blood pressure, weakness, and allergic reactions.[2] It is unclear if use in pregnancy is safe for the fetus.[2] Pyridostigmine is an acetylcholinesterase inhibitor in the cholinergic family of medications.[2] It works by blocking the action of acetylcholinesterase and therefore increases the levels of acetylcholine.[2]
Pyridostigmine was patented in 1945 and came into medical use in 1955.[3] It is on the World Health Organization’s List of Essential Medicines.[4] Pyridostigmine is available as a generic medication.[2]
Medical uses
Pyridostigmine is used to treat muscle weakness in people with myasthenia gravis or forms of congenital myasthenic syndrome and to combat the effects of curariform drug toxicity. Pyridostigmine bromide has been FDA approved for military use during combat situations as an agent to be given prior to exposure to the nerve agent Soman in order to increase survival. Used in particular during the first Gulf War, pyridostigmine bromide has been implicated as a causal factor in Gulf War syndrome.[5]
Pyridostigmine sometimes is used to treat orthostatic hypotension.[6] It may also be of benefit in chronic axonal polyneuropathy.[7]
It is also being prescribed ‘off-label’ for the postural tachycardia syndrome as well as complications resulting from Ehlers–Danlos syndrome.[7][8]
Contraindications
Pyridostigmine bromide is contraindicated in cases of mechanical intestinal or urinary obstruction and should be used with caution in patients with bronchial asthma.[9][10]
Side effects
Common side effects include:[9]
- Sweating
- Diarrhea
- Nausea
- Vomiting
- Abdominal cramps
- Increased salivation
- Tearing
- Increased bronchial secretions
- Constricted pupils
- Facial flushing due to vasodilation
- Erectile dysfunction
Additional side effects include:[9]
- Muscle twitching
- Muscle cramps and weakness
Mechanism of action
Pyridostigmine inhibits acetylcholinesterase in the synaptic cleft, thus slowing down the hydrolysis of acetylcholine. It is a quaternary carbamate inhibitor of cholinesterase that does not cross the blood–brain barrier which carbamylates about 30% of peripheral cholinesterase enzyme. The carbamylated enzyme eventually regenerates by natural hydrolysis and excess ACh levels revert to normal.
The ACh diffuses across the synaptic cleft and binds to receptors on the post synaptic membrane, causing an influx of Na+, resulting in depolarization. If large enough, this depolarization results in an action potential. To prevent constant stimulation once the ACh is released, an enzyme called acetylcholinesterase is present in the endplate membrane close to the receptors on the post synaptic membrane, and quickly hydrolyses ACh.
Names
Pyridostigmine bromide is available under the trade names Mestinon (Valeant Pharmaceuticals), Regonol and Gravitor (SUN Pharma).
Chemistry
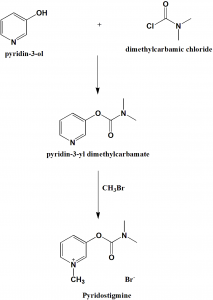
Pyridostigmine, 3-[(dimethylaminocarbonyl)oxy]-1-methyl pyridinium bromide, is synthesized from 3-hydroxypyridine, which is reacted with dimethylaminocarbamoyl chloride, which gives 3-(dimethylaminocarbamoyl)pyridine. The last is reacted with methylbromide, giving pyridostigmine.
- R. Urban, U.S. Patent 2,572,579 (1951).
Syn
youtube
SYN
Method of synthesis
i. 3-hydroxypiridine is reacted with dimethylaminocarbamoyl chloride to give 3-(dimethylaminocarbamoyl)pyridine.
ii. The above formed compound is reacted with methylbromide to produce pyridostigmine. [2]

CLIP

Paper
Journal of Biological Chemistry (1961), 236, 1498-500.
Zeitschrift fuer Klinische Medizin (1985) (1986), 41(7), 495-8
Zhonghua Yaoxue Zazhi (1993), 45(6), 601-14.
Trends in Organic Chemistry (2011), 15, 25-31.
PATENT
WO 9822458
PATENT
WO 2008074816
https://patents.google.com/patent/WO2008074816A1/en
References
- ^ World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 429. hdl:10665/44053. ISBN 9789241547659.
- ^ Jump up to:a b c d e f g h i “Neostigmine Bromide”. The American Society of Health-System Pharmacists. Archived from the original on 21 December 2016. Retrieved 8 December 2016.
- ^ Fischer, Janos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 540. ISBN 9783527607495. Archived from the original on 2016-12-20.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Golomb BA (March 2008). “Acetylcholinesterase inhibitors and Gulf War illnesses”. Proceedings of the National Academy of Sciences of the United States of America. 105 (11): 4295–300. Bibcode:2008PNAS..105.4295G. doi:10.1073/pnas.0711986105. JSTOR 25461411. PMC 2393741. PMID 18332428. Lay summary – Reuters (March 10, 2008).
- ^ Gales BJ, Gales MA (2007). “Pyridostigmine in the treatment of orthostatic intolerance”. Annals of Pharmacotherapy. 41 (2): 314–8. doi:10.1345/aph.1H458. PMID 17284509. S2CID 22855759.
- ^ Jump up to:a b Gales BJ, Gales MA (February 2007). “Pyridostigmine in the treatment of orthostatic intolerance”. The Annals of Pharmacotherapy. 41 (2): 314–8. doi:10.1345/aph.1H458. PMID 17284509. S2CID 22855759.
- ^ Kanjwal K, Karabin B, Sheikh M, et al. (June 2011). “Pyridostigmine in the treatment of postural orthostatic tachycardia: a single-center experience”. Pacing and Clinical Electrophysiology. 34 (6): 750–5. doi:10.1111/j.1540-8159.2011.03047.x. PMID 21410722. S2CID 20405336.
- ^ Jump up to:a b c Mestinon | Home Archived 2008-05-13 at the Wayback Machine
- ^ Mestinon Official FDA information, side effects and uses Archived 2008-05-24 at the Wayback Machine
External links[
- “Pyridostigmine”. Drug Information Portal. U.S. National Library of Medicine.
| Clinical data | |
|---|---|
| Trade names | Mestinon, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682229 |
| Pregnancy category | AU: C |
| Routes of administration | by mouth, intravenous |
| ATC code | N07AA02 (WHO) |
| Legal status | |
| Legal status | UK: POM (Prescription only)US: ℞-only |
| Pharmacokinetic data | |
| Bioavailability | 7.6 +/- 2.4% |
| Elimination half-life | 1.78 +/- 0.24hrs |
| Excretion | kidney |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 155-97-5 |
| PubChem CID | 4991 |
| DrugBank | DB00545 |
| ChemSpider | 4817 |
| UNII | 19QM69HH21 |
| KEGG | D00487 |
| ChEMBL | ChEMBL1115 |
| CompTox Dashboard (EPA) | DTXSID20165786 |
| Chemical and physical data | |
| Formula | C9H13N2O2 |
| Molar mass | 181.215 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| hideSMILESO=C(Oc1ccc[n+](c1)C)N(C)C | |
| hideInChIInChI=1S/C9H13N2O2/c1-10(2)9(12)13-8-5-4-6-11(3)7-8/h4-7H,1-3H3/q+1 Key:RVOLLAQWKVFTGE-UHFFFAOYSA-N |
/////////////Pyridostigmine,