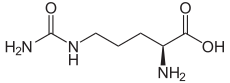
CITRULLINE
CAS 372-75-8
- L-Citrulline
- 瓜氨酸
Used for nutritional supplementation, also for treating dietary shortage or imbalance.
L-Citrulline
- Molecular FormulaC6H13N3O3
- Average mass175.186 Da
SYN
Hua Bai, Peijie Yang, Zhengjie Chen, Chongyan Xu, Zhaorul Li, Zigang Zhao, Luyan Jiang, Zongyi Yang, Jiang Li, “PROCESSES FOR THE PRODUCTION OF L-CITRULLINE.” U.S. Patent US20090142813, issued June 04, 2009.
US20090142813(S)-2-Amino-5-ureidopentanoic acid1725416[Beilstein]206-759-6[EINECS]372-75-8[RN]a-Amino-d-ureidovaleric Acid
Product Ingredients
| INGREDIENT | UNII | CAS | INCHI KEY |
|---|---|---|---|
| Citrulline malate | PAB4036KHO | 70796-17-7 | DROVUXYZTXCEBX-WCCKRBBISA-N |
CitrullineCAS Registry Number: 372-75-8
CAS Name:N5-(Aminocarbonyl)-L-ornithine
Additional Names: d-ureidonorvaline; a-amino-d-ureidovaleric acid; Nd-carbamylornithine
Molecular Formula: C6H13N3O3Molecular Weight: 175.19
Percent Composition: C 41.13%, H 7.48%, N 23.99%, O 27.40%Line Formula: H2NCONH(CH2)3CH(NH2)COOH
Literature References: An amino acid, first isolated from the juice of watermelon, Citrullus vulgaris Schrad., Cucurbitaceae: Wada, Biochem. Z.224, 420 (1930); isoln from casein: Wada, ibid.257, 1 (1933). Synthesis from ornithine through copper complexes: Kurtz, J. Biol. Chem.122, 477 (1938); by alkaline hydrolysis of arginine: Fox, ibid.123, 687 (1938); from cyclopentanone oxime: Fox et al.,J. Org. Chem.6, 410 (1941). Crystallization: Matsuda et al.,JP71 174 (1971 to Ajinomoto), C.A.74, 126056u (1971). Crystal and molecular structure: Naganathan, Venkatesan, Acta Crystallogr.27B, 1079 (1971); Ashida et al.,ibid.28B, 1367 (1972). Use in asthenia and hepatic insufficiency: FR2198739 (1974 to Hublot & Vallet), C.A.82, 144952c (1975). Clinical trial in treatment of lysinuric protein intolerance: J. Rajantie et al.,J. Pediatr.97, 927 (1980); T. O. Carpenter et al.,N. Engl. J. Med.312, 290 (1985).Properties: Prisms from methanol + water, mp 222°. [a]D20 +3.7° (c = 2). pK1 2.43; pK2 9.41. Sol in water. Insol in methanol, ethanol.Melting point: mp 222°pKa: pK1 2.43; pK2 9.41Optical Rotation: [a]D20 +3.7° (c = 2) Derivative Type: HydrochlorideCAS Registry Number: 34312-10-2Molecular Formula: C6H13N3O3.HClMolecular Weight: 211.65Percent Composition: C 34.05%, H 6.67%, N 19.85%, O 22.68%, Cl 16.75%Properties: Crystals, dec 185°. [a]D22 +17.9° (c = 2).Optical Rotation: [a]D22 +17.9° (c = 2) Derivative Type: Malate (salt)CAS Registry Number: 54940-97-5Trademarks: Stimol (Biocodex)Molecular Formula: C6H13N3O3.C4H6O5Molecular Weight: 309.27Percent Composition: C 38.84%, H 6.19%, N 13.59%, O 41.39% Therap-Cat: Treatment of asthenia.
Asklepion is developing an iv formulation of citrulline, Citrupress, for the potential treatment of pulmonary hypertension and for the potential prevention of clinical sequelae of acute lung injury complicating congenital heart repair surgery in pediatric patients, and also investigating the drug for the potential treatment of acute sickle cell crisis. In August 2016, a phase III study was initiated for preventing clinical sequelae of acute lung injury?in pediatric patients undergoing cardiopulmonary bypass (CPB) for heart defects; in July 2019, results were expected in October 2019.
Citrulline is an amino acid. It is made from ornithine and carbamoyl phosphate in one of the central reactions in the urea cycle. It is also produced from arginine as a by-product of the reaction catalyzed by NOS family. Its name is derived from citrullus, the Latin word for watermelon, from which it was first isolated.
The organic compound citrulline is an α-amino acid.[2] Its name is derived from citrullus, the Latin word for watermelon. Although named and described by gastroenterologists since the late 19th century, it was first isolated from watermelon in 1914 by Japanese researchers Yotaro Koga and Ryo Odake[3][note 1] and further codified by Mitsunori Wada of Tokyo Imperial University in 1930.[4] It has the formula H2NC(O)NH(CH2)3CH(NH2)CO2H. It is a key intermediate in the urea cycle, the pathway by which mammals excrete ammonia by converting it into urea. Citrulline is also produced as a byproduct of the enzymatic production of nitric oxide from the amino acid arginine, catalyzed by nitric oxide synthase.[5]
Biosynthesis
Citrulline is made from ornithine and carbamoyl phosphate in one of the central reactions in the urea cycle. It is also produced from arginine as a byproduct of the reaction catalyzed by NOS family (NOS; EC 1.14.13.39).[6] It is made from arginine by the enzyme trichohyalin at the inner root sheath and medulla of hair follicles.[7] Arginine is first oxidized into N-hydroxyl-arginine, which is then further oxidized to citrulline concomitant with release of nitric oxide.
Citrulline is also made by enterocytes of the small intestine.[2][8]
Function
Several proteins contain citrulline as a result of a posttranslational modification. These citrulline residues are generated by a family of enzymes called peptidylarginine deiminases (PADs), which convert arginine into citrulline in a process called citrullination or deimination with the help of calcium ion. Proteins that normally contain citrulline residues include myelin basic protein (MBP), filaggrin, and several histone proteins, whereas other proteins, such as fibrin and vimentin are susceptible to citrullination during cell death and tissue inflammation.
Circulating citrulline concentration is a biomarker of intestinal functionality.[9][10
PAPER
Biochemistry, 53(41), 6511-6519; 2014
PAPER
Journal of the Chemical Society of Pakistan, 34(2), 451-454; 2012

PAPER
Journal of Agricultural and Food Chemistry, 66(33), 8841-8850; 2018
https://pubs.acs.org/doi/10.1021/acs.jafc.8b02858
l-Citrulline is a nonessential amino acid with a variety of physiological functions and can be enzymatically produced by arginine deiminase (ADI, EC 3.5.3.6). The enzymatic-production approach is of immense interest because of its mild conditions, high yield, low cost, and environmental benignity. However, the major hindrances of l-citrulline industrialization are the poor thermostability and enzyme activity of ADI. Hence, in this work, directed evolution and site-directed mutagenesis aided with in silico screening, including the use of b-factor values and HoTMuSiC, were applied to a previously identified ADI from Enterococcus faecalis SK23.001 (EfADI), and a triple-site variant R15K–F269Y–G292P was obtained. The triple-site variant displays a 2.5-fold higher specific enzyme activity (333 U mg–1), a lower Km value of 6.4 mM, and a 6.1-fold longer half-life (t1/2,45°C = 86.7 min) than wild-type EfADI. This work provides a protein-engineering strategy to improve enzyme activity and thermostability, which might be transferrable to other ADIs and enzymes.

PAPER
ACS Sustainable Chemistry & Engineering, 7(9), 8522-8529; 2019
https://pubs.acs.org/doi/10.1021/acssuschemeng.9b00301
Biocatalytic transformation of carbamate formed readily from CO2 and NH3 provides attractive green routes for mitigation of these important environmental pollutants. Accordingly, a coupled-enzyme system was developed for the one-pot production of citrulline through carbamoylation of ornithine in aqueous solutions of CO2 and NH3. Hyperthermophilic ornithine carbamoyltransferases are produced recombinantly in E. coli with carbamate kinases known to have a propensity for carbamoyl phosphate synthesis. Importantly, in vitro biocatalysis is carried out by E. coli cell lysate prepared through coexpression of the required recombinant enzymes in a single bacterial culture, greatly reducing limitations normally associated with protein production and purification. Acetate kinase that is endogenous in the lysate also recycles the required ATP cofactor, which would otherwise have been required in costly stoichiometric amounts. Recombinant lysates catalyze the production of carbamoyl phosphate with substoichiometric ATP (>300 turnovers) as well as its in situ reaction with ornithine to give citrulline in high yield (>95%) and g L–1 h–1 titers. The system is active over a wide range of NH3 concentrations (2.5 mM – 2 M), and >90% conversions of NH3 may be reached within 1.5 h. Aqueous NH3 used to sequester CO2 gas (10% v/v) may be directly used as the biocatalyst feedstock. In preliminary studies, citrulline is found to be an effective organic nitrogen fertilizer of the wheat grass Brachypodium distachyon. Therefore, lysates described here constitute a cost-effective biocatalytic platform for one-pot production of a promising organic nitrogen fertilizer, under mild reaction conditions, from environmental pollutants as feedstock.

PATENT
WO 2015050276
https://patents.google.com/patent/WO2015050276A1/en
PATENT
WO2018125999 claiming method for maintaining the coupling of endothelial nitric oxide synthase.
PATENT
WO-2020247853
Process for preparing citrulline from a transition metal complex of ornithine using cyanate useful to reduce the incidence or severity of cardiopulmonary bypass-induced pulmonary injury due to free radical formation in a patient during cardiopulmonary bypass.
Ornithine is an alpha amino acid with a terminal amino group opposite the alpha carbon.
Citrulline is an alpha amino acid with a terminal carbamido group in the same position as the terminal amino group of ornithine. Dr. A. Kurtz described synthesis of racemic citrulline from racemic ornithine in 1938 (J. Biol. Chem., 122:477-484), and that disclosure was followed up by synthesis of optically active /-citrulline from /-ornithine in 1949 (J. Biol. Chem., 180: 1253-1267). Optical activity was preserved by complexing the starting material (/-ornithine) in a transition metal complex via the alpha amino and carboxyl groups, then reacting the terminal amino group with urea to from a carbamido derivative (see Figure 1). Kurth 1949 describes numerous other syntheses, all depending on the transition metal complex to preserve the alpha amino acid character of the starting compound while derivatizing other parts of the molecule. An example of this synthesis is described in Example 1 below.
Details of various steps in the improved processes developed by the present inventors for producing pharmaceutical grade citrulline are discussed below.
Synthesis of Citrulline from Ornithine
[00014] The present inventors preserved the stereochemical structure around the alpha carbon of the alpha amino acid during reaction of amino groups elsewhere on the compound by complexing the alpha end of the molecule with a transition metal atom, as reported by
Kurth 1938 and 1949. The initial production of the /-ornithine-copper complex is carried out as described by Kurtz. Kurtz describes a variety of transition metals as the complexing metal in the 1949 paper, but the preferred metal is copper (II), based on the ease of forming stable complexes and the ease with which copper (II) may subsequently be removed from the product. The copper is typically supplied as cupric sulfate, although complex formation from copper (II) acetate, cupric carbonate, or cupric oxide have also been reported.
[00015] The present inventors have discovered an alternative method of derivatizing the terminal amino group of the complexed alpha amino acid using cyanate rather than the urea reaction reported by Kurth. An example of this improved synthesis is shown in Figure 2A and described in Example 3 below. Use of cyanate as the derivatizing agent has been found to produce fewer distinct product compounds, which simplifies purification of the desired citrulline product. Kurth carried out urea derivatization by refluxing the copper complex in the presence of excess urea. Cyanate derivatization may be carried out at lower temperatures (e.g. 55°C-65°C) which may contribute to higher yield of citrulline, based on the initial amount of ornithine. Cyanate is preferably provided in excess, and the reaction is driven by precipitation of the citrulline: copper complex. The precipitated complex is washed with water to remove unreacted copper (e.g., wash until no blue coloration persists in the filtrate). The precipitated copper complex of citrulline may be recovered and dried.
Enriching Citrulline as a Copper Complex
[00016] The inventors have discovered that the relative citrulline content of the reaction
product(s) can be enhanced by reprecipitation of the citrulline: copper complex. Precipitated copper complex of citrulline (produced, for example, by reaction of a ornithine: copper complex with urea or cyanate in water) may be dried. The
citrulline: copper complex may be redissolved by suspending the precipitate in water and acidifying the suspension until the complex dissolves. Acidification may be
accomplished by adding concentrated acid, preferably hydrogen chloride, to the suspension while stirring. Once the copper: citrulline complex solution is clear, base (typically sodium hydroxide) is added to bring the pH up to 7-10. Both the acidification and subsequent neutralization steps are actively cooled (temperature not more than 45°C) to protect the citrulline product from hydrolysis or reaction to produce side products. The precipitate is washed with water (e.g., until the filtrate is free of chloride by checking the filtrate for turbidity with silver nitrate), and then the precipitate is dried. Reprecipitation under these conditions is selective for citrulline: copper complex over ornithine: copper complex, because the ornithine complex is more soluble in water. If the dried complex contains higher than the desired level of ornithine contamination (e.g., greater than 10 mole% ornithine – as measured by NMR, for example), the complex may be redissolved and reprecipitated as necessary to further lower the relative amount of ornithine.
Recovering Citrulline from Its Copper Complex
[00017] Once the ornithine content in the copper: citrulline complex precipitate is sufficiently low
(preferably less than 10 mole% ornithine), the precipitate is resuspended in water and citrulline is freed from the complex by removing the copper as an inorganic precipitate, typically copper sulfide (See Figure 2B). Sulfide may be introduced in a variety of salt forms, but the inventors have found it preferable to use hydrogen sulfide gas as the sulfide source. In a preferred mode, the aqueous suspension is placed in a stirred, pressure vessel. The air is then pumped out of the reactor’s head space to form an under pressure. The reactor is then repressurized with hydrogen sulfide gas over the aqueous suspension (preferably at low temperature, e.g., 0°C-5°C, to maximize the solubility of hydrogen sulfide). Hydrogen sulfide is continuously added to the reactor to maintain parity with ambient pressure during consumption of this gas. Copper salts will precipitate, leaving citrulline in solution. As hydrogen sulfide is consumed, the pressure in the vessel decreases; the reaction is complete when the pressure stabilizes. Reaction of hydrogen sulfide with residual copper salts (for example chloride or sulfate) will lower the pH; typically the pH will be below 4, preferably pH~3. Copper salts typically include copper (II) sulfide, but may also include copper (I) sulfide and copper oxide. The solution temperature is elevated for filtration, typically to about 30°C, to promote solubility of the citrulline and drive off excess hydrogen sulfide gas, while precipitated copper salts are removed by filtration.
Purifying Citrulline
[00018] For pharmaceutical use, the active compound must be substantially free of contaminants, and further purification steps are necessary to produce a pharmaceutical grade product. For the purposes of this invention, substantially free of contaminants is considered to include: ornithine not more than (NMT) 0.8%, individual specified impurities NMT 0.15%, individual unspecified (unknown) impurities NMT 0.1%; total related substances NMT 1.3%, and Cu not more than lOppm. For citrulline manufactured from ornithine using copper complex to protect the alpha amino acid functions, the inventors have found that desired purification after citrulline is released from the copper complex can be achieved by activated carbon adsorption of contaminants and solvent/anti- solvent crystallization of the active pharmaceutical component.
[00019] The citrulline-containing aqueous solution remaining after removal of precipitated copper salts is neutralized to stabilize the citrulline against hydrolysis, to enhance adsorption of residual copper to activated carbon, and to facilitate solvent/anti-solvent precipitation of citrulline; pH is preferably adjusted to 5.9 ± 0.2, the isoelectric point of citrulline. The neutralized citrulline solution may be passed through a nano-filter to remove any bacteria and/or bacterial cell wall fragments that contaminate the solution. The nano-filtered solution may be held in a semi-sterile reservoir for staging purposes between the subsequent purification steps. The neutralized citrulline solution is treated with activated carbon, either by mixing with carbon dust or passing the solution through an activated carbon adsorber bed. The aqueous citrulline-containing effluent from the activated carbon is mixed with an anti-solvent to induce anti-solvent crystallization. Suitable anti solvents are miscible with water, including aliphatic alcohols, such as 2-propanol, ethanol or methanol, as well as acetone. A preferred antisolvent for citrulline is acetone, when mixed with approximately two volumes of water (e.g., 1 volume of water to 1.8 volumes of acetone). Acetone is preferably pre-cooled so that the resultant suspension is 0°C- 10°C. The cooled suspension may be collected in a reservoir or processed by filtration immediately to recover the citrulline precipitate.
Microbial control:
[00020] Because citrulline synthesis and purification occur in aqueous solution, there is increased risk of microbial contamination and endotoxin accumulation in the product. Washing the citrulline: copper precipitate, and addition of H2S to acid solution minimize any accumulation of microbes. From the exposure of the complex to FES until treatment with acetone the aqueous solutions of citrulline are preferably kept in sealed vessels to limit microbial contamination and growth. Enclosing the purification steps to minimize contact with the environment and use of sterile filters to capture potential microbial contamination allows the manufacturing to be performed in an ISO 8 cleanroom. Alternatively, the final purification steps can be carried out in a sterile GMP environment of the sort used for aseptic filling of sterile dosage products (e.g., ISO Class 5/6).
[00021] If examination of the solution prior to the anti-solvent precipitation shows the amounts of microbes or endotoxin levels exceed those aceptable for injectable therapeutic compositions (e.g., 50 EU/g API, more preferably 20 EU/g), the product may be subjected to nano-filtration to remove microbes and endotoxin, before being recovered by anti-solvent precipitation and drying. The citrulline and water molecules pass through the nano-filtration membrane, but the larger bacteria and bacterial cell wall fragments are retained by the filter.
Filter press
[00022] The reaction mixtures may be pumped through a filter press to collect / remove the
suspended solids. See the general picture in Figure 3, and the attached photograph in Figure 4. The press is composed of a series of plates 1 which are then hydraulically pressed together. The hydraulic pressure ensures that the system is sealed. The suspension is then pumped through a central tube 2 where it spreads-out across several chambers 3 between the plates. The walls of the plates have a filter sheet, which allows the filtrate to flow past and exit via an internal cavity 4.
[00023] The general advantage of a filter press is that it allows a high surface area for filtration.
This effect greatly accelerates the portion-wise collection and washing of the complex and API. This system may be used to collect the copper salts after exposure to hydrogen sulfide. In the latter case, the suspension is pumped from the reactor into the press, and the filtrate may then be passed through an in-line 5 pm filter to catch any residual particulate copper, then an in-line sterile 0.2 pm filter at the entry port of a semi-sterile container for holding.
The press may be used to collect:
• Crude citrulline copper complex
• The complex after the pH-driven re-precipitation
• Precipitated copper salts (where citrulline leaves as solution in the filtrate)
• Precipitated citrulline from anti-solvent precipitation prior to drying
Semi-sterile containers
[00024] A useful semi-sterile container is basically a closed vessel equipped with a stirrer and ports for the addition and removal of liquid, and a pH meter. The container should be sterilized (e.g., treated with isopropyl alcohol solution and rinsed with water) directly prior to use and not opened during use. A sterile, air filter attached to the lid allows air to flow into the container as the liquid is being pumped out. The pH adjustment may be performed in this container, before treatment with activated carbon. The container is not particularly suitable for the long-term storage of the solutions.
Activated carbon adsorber bed
[00025] The solution may be pumped from the semi sterile container through the activated carbon bed (a column packed with granulated activated carbon) pre-flushed with argon. The liquid is then returned to the semi-sterile container via an in-line 5 pm filter and the 0.2 pm sterile filter at the entry port. If the solution is pumped in a cyclic manner with the stirrer activated for not less than 6 hours, the sterile filter acts as a“microbial scrubber” continually collecting any microbes in the solution. The activated carbon primarily removes any organic impurities and will also remove any residual dissolved copper ions. The 5 pm filter catches any carbon particles which detach from the bed.
Sterile bags
[00026] After processing in the activated carbon adsorber bed, the solution may be passed into a single use sterile bag via another sterile filter. The solution may be stored longer in the bag than in the semi-sterile container. At this point, a test for the presence of microbes and/or bacterial endotoxins can be carried out. If endotoxins are observed, then the cut off (nano-filtration) membrane may be employed. If not, the citrulline is ready to be
recovered from the solution by anti-solvent precipitation. Collection of the solution in a sterile bag allows the citrulline solution to be processed batch-wise, where conveniently sized portions of citrulline are precipitated and recovered in the filter press.
Solvent/Anti-solvent Mixing
[00027] The aqueous citrulline solution is mixed with pre-cooled anti-solvent to precipitate the citrulline from solution. After mixing with anti-solvent, the threat posed by bacterial growth is not higher than that for other APIs. The addition of the organic solvent makes the resulting solution bacteriostatic at a minimum. This precipitation improves the purity of citrulline, reducing, in particular, the ornithine levels, and allows for the rapid extraction of citrulline from solution.
Final drying
[00028] The precipitate is dried to remove residual acetone and water. Drying may be carried-out in a conical dryer, firstly to drive off the acetone anti-solvent, then moisture and finally the water of crystallization. The conical dryer can also be used to homogenize the product. The final, dry product of anti-solvent precipitation may be stored, and ultimately dissolved in sterile aqueous diluent for therapeutic administration.
[00029] On dissolution in sterile aqueous media, citrulline prepared as described herein may be used to treat pulmonary hypertension (WO/2000/073322), bronchopulmonary dysplasia (WO/2009/099998), sickle cell crisis (WO/2018/157137), cardiac surgery patients (WO/2005/082042), cardiopulmonary bypass patients (WO/2018/125999), and vasospasm as a complication of subarachnoid hemorrhage (WO/2009/099999), by parenteral administration as described in these documents, incorporated herein by reference.
EXAMPLES
Example 1. Synthesis of citrulline from ornithine using urea.
[00030] L-Citrulline is synthesized from L-omithine and urea. A flow chart of the reaction is shown in Figure 1 A.
[00031] L-Citrulline is prepared synthetically starting from L-ornithine hydrochloride. Into a 120- L reactor containing approximately 50 liters of water, 10 kilograms of L-omithine hydrochloride is added and dissolved. The solution is neutralized with potassium hydroxide and then converted to its copper complex by the addition of 15kg copper sulfate (molar equivalent amount). The copper complex protects the 2-amino carboxylic acid functionality in the molecule while chemistry is performed on the terminal amino group. The L-ornithine copper complex is then exposed to an excess of urea at reflux, which promotes its conversion to the copper complex of L-citrulline. The resulting copper complex of L-citrulline then is precipitated and collected by filtration.
[00032] The isolated copper complex of L-citrulline is dried and testing is performed. The
appearance is verified, and an in-use performance test is done to determine suitability to proceed.
Example 2. Purification of citrulline from copper-citrulline complex.
[00033] L-Citrulline synthesized from L-ornithine and urea is purified by resin-based purification and recrystallization. A flow chart of the reaction is shown in Figure IB.
[00034] In a 120-L reactor, ~13 kilograms of the L-citrulline copper complex prepared in
Example 1 is added to a stirring solution of sodium sulfide (Na2S) in water
(approximately 8 kilograms Na2S in 50 liters of water), causing the precipitation of copper sulfide and the freeing of L-citrulline. The solution is filtered to remove the copper salts. The pH of the resulting aqueous solution containing the sodium salt of L- citrulline and residual sodium sulfide is lowered to 4 by the addition of an acidic ion exchange resin (such as Amberlite ). A constant stream of argon gas is passed through the solution to remove the residual sulfide as hydrogen disulfide. The pH of the solution is then raised to 5.9 ± 0.2 using sodium hydroxide to form isoelectric L-citrulline.
). A constant stream of argon gas is passed through the solution to remove the residual sulfide as hydrogen disulfide. The pH of the solution is then raised to 5.9 ± 0.2 using sodium hydroxide to form isoelectric L-citrulline.
Activated carbon is then added to the reaction mixture to remove residual impurities, in particular residual copper ions. The solids (Amberlite and activated carbon) are then removed by filtration, and the filtrate is concentrated to approximately 50 liters (either by evaporation or reverse osmosis). L-citrulline is then precipitated from the aqueous solution by the addition of an equal part of acetone, and the mixture is cooled to near 0°C. The precipitate is collected by filtration and dried in a vacuum oven.
and activated carbon) are then removed by filtration, and the filtrate is concentrated to approximately 50 liters (either by evaporation or reverse osmosis). L-citrulline is then precipitated from the aqueous solution by the addition of an equal part of acetone, and the mixture is cooled to near 0°C. The precipitate is collected by filtration and dried in a vacuum oven.
[00035] The non-sterile bulk powder is then reconstituted and processed for endotoxin reduction and sterile filtration steps followed by crystallization, drying and micronization in an aseptic environment. The sterile bulk powder is then used as the“raw material” for aseptic filling into glass vials to produce the finished drug product which may be reconstituted with a sterile diluent prior to use.
Example 3. Synthesis of citrulline from ornithine using cyanate
[00036] L-Citrulline was prepared synthetically starting from L-omithine hydrochloride. Into a reactor containing sodium hydroxide (11 kg) in water (170 kg), L-ornithine hydrochloride (44 kg) was added and dissolved. The temperature was maintained at no more than 40°C by active cooling. The ornithine was then converted to its copper complex by the addition of 0.5 molar equivalents of copper sulfate (33 kg) and stirring at ambient temperature for more than 15 minutes. The copper complex protects the 2-amino carboxylic acid functionality of the molecule while chemistry is performed on the terminal amino group. A molar excess of potassium cyanate (32 kg) is then added to the L-ornithine copper complex, and the solution is held at 55°C-65°C for 4.0-4.5 hours, which promotes its conversion to the copper complex of L-citrulline. The resulting copper complex of L-citrulline precipitates during the reaction, and it is collected by filtration.
Example 4. Purification of therapeutic grade citrulline.
[00037] The dry copper: citrulline complex produced in Example 3 is added to a reactor
containing water, which is stirred to resuspend the complex. Concentrated hydrogen chloride solution is added to convert the complex into a solution of copper (II) chloride and citrulline hydrochloride, while the temperature of the reactor is maintained at no more than 45°C by active cooling. Once the contents of the reactor are in solution, sodium hydroxide is added to raise the pH to 7-10, while the temperature is maintained at no more than 40°C. The copper complex of citrulline then precipitates. The precipitate is collected and washed with water until no blue coloration persists in the filtrate.
[00038] The washed precipitate is tested to determine the relative ornithine content. If ornithine is greater than 10 mole%, the precipitate is redissolved and resuspended as described above, until the ornithine content is lowered to not more than 10 mole%.
[00039] Once the precipitate achieves the desired ornithine content, it is resuspended in water in a stirred reactor, and hydrogen sulfide gas is introduced into the suspension to precipitate copper sulfide and dissolve citrulline. The solution is warmed to 30°C ± 2°C to ensure citrulline is fully solubilized, and precipitated copper salts are removed by filtration. The citrulline-containing filtrate is passed thorough micro- and sterile-filtrations and collected in a semi-sterile reactor.
[00040] Activated carbon is used to remove residual impurities, in particular an organic
component and residual copper ions. The pH of the resulting aqueous solution containing L-citrulline and residual copper is adjusted to 5.9 ± 0.2 with sodium hydroxide to form isoelectric citrulline solution. The isoelectric citrulline solution is treated with active carbon granules, preferably by passing the solution through an active carbon adsorber bed, and passed through micro and sterile filters after the active carbon treatment.
[00041] L-citrulline is then precipitated from the aqueous solution by the addition of acetone anti solvent, and the mixture is cooled to near 0°C. Addition of 1.5 to 2 volume equivalents of acetone produce dihydrate crystals of citrulline. The precipitate is collected by filtration. The crystals are dried in a vacuum in a conical dryer at temperature of no more than 45°C to remove acetone and water, resulting in an anhydrous crystalline solid. This solid citrulline corresponds to the orthorhombic d form anhydrous crystals reported by Allouchi, et al., 2014 ( Cryst . Growth Des., 14: 1279-1286).
[00042] Either the dihydrate crystals or the anhydrous crystals may be used therapeutically. The solid or an aqueous solution/suspension may be administered enterally, or the solid may be redissolved for parenteral administration. To produce a final therapeutic product, the non-sterile bulk powder was reconstituted and underwent endotoxin reduction and sterile filtration steps followed by crystallization, drying and micronization in an aseptic environment. The sterile bulk powder was then used as the“raw material” for aseptic filling into glass vials to produce the finished drug product which was reconstituted with a sterile diluent prior to use.
References
- ^ “Citrulline – Compound Summary”. PubChem Compound. USA: National Center for Biotechnology Information. 16 September 2004. Identification. Retrieved 1 May 2012.
- ^ Jump up to:a b Banerjee, Aryamitra (2014-01-01), Gupta, Ramesh C. (ed.), “Chapter 15 – Gastrointestinal toxicity biomarkers”, Biomarkers in Toxicology, Boston: Academic Press, pp. 269–277, doi:10.1016/b978-0-12-404630-6.00015-4, ISBN 978-0-12-404630-6, retrieved 2020-11-10
- ^ Fragkos, Konstantinos C.; Forbes, Alastair (September 2011). “Was citrulline first a laxative substance? The truth about modern citrulline and its isolation” (PDF). Nihon Ishigaku Zasshi. [Journal of Japanese History of Medicine]. 57 (3): 275–292. ISSN 0549-3323. PMID 22397107.
- ^ Fearon, William Robert (1939). “The Carbamido Diacetyl Reaction: A Test For Citrulline”. Biochemical Journal. 33 (6): 902–907. doi:10.1042/bj0330902. PMC 1264464. PMID 16746990.
- ^ “Nos2 – Nitric Oxide Synthase”. Uniprot.org. Uniprot Consortium. Retrieved 10 February 2015.
- ^ Cox M, Lehninger AL, Nelson DR (2000). Lehninger principles of biochemistry (3rd ed.). New York: Worth Publishers. p. 449. ISBN 978-1-57259-153-0. Retrieved 13 March 2020.
- ^ Rogers, G. E.; Rothnagel, J. A. (1983). “A sensitive assay for the enzyme activity in hair follicles and epidermis that catalyses the peptidyl-arginine-citrulline post-translational modification”. Current Problems in Dermatology. 11: 171–184. doi:10.1159/000408673. ISBN 978-3-8055-3752-0. PMID 6653155.
- ^ DeLegge, Mark H. (2019-01-01), Corrigan, Mandy L.; Roberts, Kristen; Steiger, Ezra (eds.), “Chapter 7 – Enteral Access and Enteral Nutrition in Patients With Short Bowel Syndrome”, Adult Short Bowel Syndrome, Academic Press, pp. 81–96, doi:10.1016/b978-0-12-814330-8.00007-x, ISBN 978-0-12-814330-8, retrieved 2020-11-10
- ^ Fragkos, Konstantinos C.; Forbes, Alastair (2017-10-12). “Citrulline as a marker of intestinal function and absorption in clinical settings: A systematic review and meta-analysis”. United European Gastroenterology Journal. 6 (2): 181–191. doi:10.1177/2050640617737632. PMC 5833233. PMID 29511548.
- ^ Crenn, P.; et al. (2000). “Post-absorptive plasma citrulline concentration is a marker of intestinal failure in short bowel syndrome patients”. Gastroenterology. 119 (6): 1496–505. doi:10.1053/gast.2000.20227. PMID 11
///////CITRULLINE, L-Citrulline, 瓜氨酸 ,
