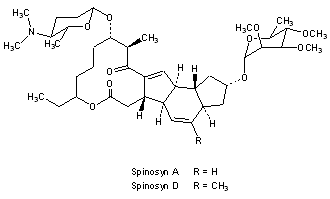


Spinosad
Spinosyn A: The chemical name is: 1H-as-Indaceno[3,2- d]oxacyclododecin-7,a5-dione, 2-[(6-deoxy-2,3,4-tri-O-methyl-alphaL-mannopyranosyl)oxy]-13-[[2R,5S,6R)-5-(dimethylamino) tetrahydro-6-methyl-2H-pyran-2-yl]oxy]-9-ethyl2,3,3a,5a,5b,6,9,10,11,12,13,14,16a,16b-tetradecahydro-14-metyl-, (2R,3aS,5aR,5bS,9S,13S,14R,16aS,16bR)-
Spinosyn D: The chemical name is: 1H-as-Indaceno[3,2- d]oxacyclododecin-7,15-dione, 2-[(6-deoxy-2,3,4-tri-O-methyl-alphaL-mannopyranosyl)oxy]-13-[[2R,5S,6R)-5-(dimethylamino) tetrahydro-6-methyl-2H-pyran-2-yl]oxy]-9-ethyl2,3,3a,5a,5b,6,9,10,11,12,13,14,16a,16b-tetradecahydro-4,14-dimetyl-, (2S,3aSR,5aS,5bS,9S,13S,14R,16aS,16bS)-
168316-95-8
- Molecular FormulaC83H132N2O20
- Average mass1477.938 Da
- Comfortis
- Conserve
- EC 434-300-1
- Natroba
- NaturaLyte
- Spinosad
- Tracer
- Tracer Naturalyte
- UNII-XPA88EAP6V
- XDE 105
Natroba (Spinosad) Suspension 0.9% ParaPro Pharma
New Drug Application (NDA): 022408 appr 01/18/2011
spinosad, is a new molecular entity, and a fermentation product produced by the actinomycete, Saccharopolyspora spinosa. Spinosad contains two components, spinosyn A and D. T

Figure 1. Structure of spinosyn A and DTitle: SpinosynsCAS Registry Number: 131929-60-7Literature References: Class of fermentation derived 12 membered macrocyclic lactones in a unique tetracyclic ring. At least 20 spinosyns have been isolated from Saccharopolyspora spinosa; variations in the two sugars account for most of the structural and insecticidal activity differences. Isolation and biological activity: L. D. Boeck et al.,EP375316 (1990 to Lilly); eidem,US5496931 (1996 to DowElanco); and structure determn: H. A. Kirst et al.,Tetrahedron Lett.32, 4839 (1991). Soil degradation: K. A. Hale, D. E. Portwood, J. Environ. Sci. HealthB31, 477 (1996). HPLC determn in vegetables: L.-T. Yeh et al.,J. Agric. Food Chem.45, 1746 (1997); in soil and water: S. D. West, ibid. 3107. Uptake and metabolism in larvae: T. C. Sparks et al.,Proc. Beltwide Cotton Conf.2, 1259 (1997). Mode of action study: V. L. Salgado et al.,Pestic. Biochem. Physiol.60, 103 (1998). Review of physical and biological properties: C. V. DeAmicis et al.,ACS Symp. Ser.658, 144-154 (1997). Review: G. D. Crouse, T. C. Sparks, Rev. Toxicol.2, 133-146 (1998).
Derivative Type: Spinosyn Acas 131929-60-7CAS Name: (2R,3aS,5aR,5bS,9S,13S,14R,16aS,16bR)-2-[(6-Deoxy-2,3,4-tri-O-methyl-a-L-mannopyranosyl)oxy]-13-[[(2R,5S,6R)-5-(dimethylamino)tetrahydro-6-methyl-2H-pyran-2-yl]oxy]-9-ethyl-2,3,3a,5a,5b,6,9,10,11,12,13,14,16a,16b-tetradecahydro-14-methyl-1H-as-indaceno[3,2-d]oxacyclododecin-7,15-dioneAdditional Names: lepicidin AManufacturers’ Codes: A-83543A; LY-232105Molecular Formula: C41H65NO10Molecular Weight: 731.96Percent Composition: C 67.28%, H 8.95%, N 1.91%, O 21.86%Literature References: Total synthesis: L. A. Paquette et al.,J. Am. Chem. Soc.120, 2553 (1998).Properties: White, odorless crystalline solid, mp 118°. pKa 8.1. uv max (methanol): 243 nm (e 11000). [a]27436 -262.7° (methanol). Vapor pressure: 2.4 ´ 10-10. Soly in water (ppm): 290 (pH 5), 235 (pH 7), 16 (pH 9), distilled 20. Soly (w/v%): methanol 19, acetone 17, dichloromethane >50, hexane 0.45%. LD50 in rats (mg/kg): 3783-5000 orally (Crouse).Melting point: mp 118°pKa: pKa 8.1Optical Rotation: [a]27436 -262.7° (methanol)Absorption maximum: uv max (methanol): 243 nm (e 11000)Toxicity data: LD50 in rats (mg/kg): 3783-5000 orally (Crouse)
Derivative Type: Spinosyn DCAS Registry Number: 131929-63-0Manufacturers’ Codes: A-83543DMolecular Formula: C42H67NO10Molecular Weight: 745.98Percent Composition: C 67.62%, H 9.05%, N 1.88%, O 21.45%Properties: Odorless, white crystalline solid. mp 169°. pKa 7.8. uv max (methanol): 243 nm (e 11000). [a]27436 -297.5° (methanol). Vapor pressure: 2.0 ´ 10-10. Soly in water (ppm): 28 (pH 5), 0.329 (pH 7), 0.04 (pH 9), distilled 1.3. Soly (w/v%): methanol 0.25, acetone 1.0, dichloromethane 45, hexane 0.07%.Melting point: mp 169°pKa: pKa 7.8Optical Rotation: [a]27436 -297.5° (methanol)Absorption maximum: uv max (methanol): 243 nm (e 11000)
Derivative Type: SpinosadCAS Registry Number: 168316-95-8Manufacturers’ Codes: XDE-105; DE-105Trademarks: Conserve (Dow AgroSci.); Justice (Dow AgroSci.); Naturalyte (Dow AgroSci.); SpinTor (Dow AgroSci.); Success (Dow AgroSci.); Tracer (Dow AgroSci.)Literature References: Mixture of spinosyns A and D. Effect on beneficial insects: D. Murray, R. Lloyd, Australian Cottongrower18, 62 (1997).Properties: Light grey to white crystals (tech). LD50 in rats, mallard ducks, quail (mg/kg): >3600, >2000, >2000 orally (Crouse).Toxicity data: LD50 in rats, mallard ducks, quail (mg/kg): >3600, >2000, >2000 orally (Crouse)
Use: Insecticide.(2R,3aS,5aR,5bS,9S,13S,14R,16aS,16bR)-13-{[(2R,5S,6R)-5-(Dimethylamino)-6-methyltetrahydro-2H-pyran-2-yl]oxy}-9-ethyl-14-methyl-7,15-dioxo-2,3,3a,5a,5b,6,7,9,10,11,12,13,14,15,16a,16b-hexadecahydro-1H ;-as-indaceno[3,2-d]oxacyclododecin-2-yl 6-deoxy-2,3,4-tri-O-methyl-α-L-mannopyranoside – (2S,3aR,5aS,5bS,9S,13S,14R,16aS,16bS)-13-{[(2R,5S,6R)-5-(dimethylamino)-6-methyltetrahydro-2H-pyran-2-yl]ox y}-9-ethyl-4,14-dimethyl-7,15-dioxo-2,3,3a,5
1H-as-Indaceno[3,2-d]oxacyclododecin-7,15-dione, 2-[(6-deoxy-2,3,4-tri-O-methyl-α-L-mannopyranosyl)oxy]-13-[[(2R,5S,6R)-5-(dimethylamino)tetrahydro-6-methyl-2H-pyran-2-yl]oxy]-9-ethyl-2,3,3a,5a,5b ,6,9,10,11,12,13,14,16a,16b-tetradecahydro-14-methyl-, (2R,3aS,5aR,5bS,9S,13S,14R,16aS,16bR)-, compd. with (2S,3aR,5aS,5bS,9S,13S,14R,16aS,16bS)-2-[(6-deoxy-2,3,4-tri-O-methyl-α-L-mannopyranosyl)o xy]-13-[[(2R,5S,6R)-5-(dimethylamino)tetrahySpinosad[USAN] [Wiki]168316-95-8 [RN]1H-as-Indaceno[3,2-d]oxacyclododecin-7,15-dione, 2-[(6-deoxy-2,3,4-tri-O-methyl-α-L-mannopyranosyl)oxy]-13-[[2R,5S,6R)-5-(dimethylamino) tetrahydro-6-methyl-2H-pyran-2-yl]oxy]-9-ethyl-2,3,3a,5a,5b,6,9,10,11,12,13,14,16a,16b-tetradecahydro-4,14-dimetyl-,(2S,3aSR,5aS,5bS,9S,13S,14R,16aS,16bS)-1H-as-Indaceno[3,2-d]oxacyclododecin-7,a5-dione, 2-[(6-deoxy-2,3,4-tri-O-methyl-α-L-mannopyranosyl)oxy]-13-[[2R,5S,6R)-5-(dimethylamino) tetrahydro-6-methyl-2H-pyran-2-yl]oxy]-9-ethyl-2,3,3a,5a,5b,6,9,10,11,12,13,14,16a,16b-tetradecahydro-14-metyl-, (2R,3aS,5aR,5bS,9S,13S,14R,16aS,16bR)-NAF-144Spinosad|spinosyn A and D (mixture)spinosyn A and D (mixture)
Spinosad is an insecticide based on chemical compounds found in the bacterial species Saccharopolyspora spinosa. The genus Saccharopolyspora was discovered in 1985 in isolates from crushed sugarcane. The bacteria produce yellowish-pink aerial hyphae, with bead-like chains of spores enclosed in a characteristic hairy sheath.[1] This genus is defined as aerobic, Gram-positive, nonacid-fast actinomycetes with fragmenting substrate mycelium. S. spinosa was isolated from soil collected inside a nonoperational sugar mill rum still in the Virgin Islands. Spinosad is a mixture of chemical compounds in the spinosyn family that has a generalized structure consisting of a unique tetracyclic ring system attached to an amino sugar (D-forosamine) and a neutral sugar (tri-Ο-methyl-L-rhamnose).[2] Spinosad is relatively nonpolar and not easily dissolved in water.[3]
Spinosad is a novel mode-of-action insecticide derived from a family of natural products obtained by fermentation of S. spinosa. Spinosyns occur in over 20 natural forms, and over 200 synthetic forms (spinosoids) have been produced in the lab.[4] Spinosad contains a mix of two spinosoids, spinosyn A, the major component, and spinosyn D (the minor component), in a roughly 17:3 ratio.[1
Mode of action
Spinosad is highly active, by both contact and ingestion, in numerous insect species.[5] Its overall protective effect varies with insect species and life stage. It affects certain species only in the adult stage, but can affect other species at more than one life stage. The species subject to very high rates of mortality as larvae, but not as adults, may gradually be controlled through sustained larval mortality.[5] The mode of action of spinosoid insecticides is by a neural mechanism.[6] The spinosyns and spinosoids have a novel mode of action, primarily targeting binding sites on nicotinic acetylcholine receptors (nAChRs) of the insect nervous system that are distinct from those at which other insecticides have their activity. Spinosoid binding leads to disruption of acetylcholine neurotransmission.[2] Spinosad also has secondary effects as a γ-amino-butyric acid (GABA) neurotransmitter agonist.[2] It kills insects by hyperexcitation of the insect nervous system.[2] Spinosad so far has proven not to cause cross-resistance to any other known insecticide.[7]
Use
Spinosad has been used around the world for the control of a variety of insect pests, including Lepidoptera, Diptera, Thysanoptera, Coleoptera, Orthoptera, and Hymenoptera, and many others.[8] It was first registered as a pesticide in the United States for use on crops in 1997.[8] Its labeled use rate is set at 1 ppm (1 mg a.i./kg of grain) and its maximum residue limit (MRL) or tolerance is set at 1.5 ppm. Spinosad’s widespread commercial launch was deferred, awaiting final MRL or tolerance approvals in a few remaining grain-importing countries. It is considered a natural product, thus is approved for use in organic agriculture by numerous nations.[5] Two other uses for spinosad are for pets and humans. Spinosad has recently been used in oral preparations (as Comfortis) to treat C. felis, the cat flea, in canines and felines; the optimal dose set for canines is reported to be 30 mg/kg.[2]
Spinosad is sold under the trade names, Comfortis, Trifexis, and Natroba.[9][10] Trifexis also includes milbemycin oxime. Comfortis and Trifexis brands treat adult fleas on pets; the latter also prevents heartworm disease. Natroba is sold for treatment of human head lice. Spinosad is also commonly used to kill thrips.[11][12][13]
Spinosyn A
Spinosyn A does not appear to interact directly with known insecticidal-relevant target sites, but rather acts via a novel mechanism.[6] Spinosyn A resembles a GABA antagonist and is comparable to the effect of avermectin on insect neurons.[4] Spinosyn A is highly active against neonate larvae of the tobacco budworm, Heliothis virescens, and is slightly more biologically active than spinosyn D. In general, spinosyns possessing a methyl group at C6 (spinosyn D-related analogs) tend to be more active and less affected by changes in the rest of the molecule.[7] Spinosyn A is slow to penetrate to the internal fluids of larvae; it is also poorly metabolized once it enters the insect.[7] The apparent lack of spinosyn A metabolism may contribute to its high level of activity, and may compensate for the slow rate of penetration.[7]
Safety and ecotoxicology
Spinosad has high efficacy, a broad insect pest spectrum, low mammalian toxicity, and a good environmental profile, a unique feature of the insecticide compared to others currently used for the protection of grain products.[5] It is regarded as natural product-based, and approved for use in organic agriculture by numerous national and international certifications.[8] Spinosad residues are highly stable on grains stored in bins, with protection ranging from 6 months to 2 years.[5][clarification needed] Ecotoxicology parameters have been reported for spinosad, and are:[14]
- in rat (Rattus norvegicus Bergenhout, 1769), acute oral: LD50 >5000 mg/kg (nontoxic)
- in rat (R. norvegicus), acute dermal: LD50 >2000 mg/kg (nontoxic)
- in California quail (Callipepla californica Shaw, 1798), oral toxicity: LD50 >2000 mg/kg (nontoxic)
- in duck (Anas platyrhynchos domestica Linnaeus, 1758), dietary toxicity: LC50 >5000 mg/kg (nontoxic)
- in rainbow trout (Oncorhynchus mykiss Walbaum, 1792), LC50-96h = 30.0 mg/l (slightly toxic)
- in Honeybee (Apis mellifera Linnaeus, 1758), LD50 = 0.0025 mg/bee (highly toxic if directly sprayed on and of dried residues).
Chronic exposure studies failed to induce tumor formation in rats and mice; mice given up to 51 mg/kg/day for 18 months resulted in no tumor formation.[15] Similarly, administration of 25 mg/kg/day to rats for 24 months did not result in tumor formation.[16]
syn
EP 0375,316 (1994, to DowElanco)
US 5496931 (1996 to DowElanco)
PATENT
CN 102190694
https://patents.google.com/patent/CN102190694A/en
Pleocidin compounds (spinosyns) is soil actinomycete thorn many armfuls of bacterium Saccharopolysporaspinosa of sugar secondary metabolites behind aerobic fermentation under developing medium.Pleocidin belongs to macrolides compound, it comprises one a plurality of chiral carbon tetracyclic ring systems (Macrolide tetracycle), big ring is gone up the 9-hydroxyl and is being linked two different hexa-atomic sugar respectively with the 17-hydroxyl, wherein that 17 connections is an aminosugar (Forosamine sugar), and that connect on the 9-position is a rhamnosyl (Rhamnose sugar).Tetracyclic ring system is by one 5,, 6,5-is suitable-and anti–anti–three-loop system condenses one 12 membered macrolide to be formed, and wherein contains an alpha, beta-unsaturated ketone and an independently two key.When 6 on ring is pleocidin A when being substituted by hydrogen, in mixture, account for 85-90%, when ring 6 bit substituents when connecing methyl, be pleocidin D then, in mixture, account for about 10-15%.Up to the present B, C, D, E, F, G, K, L, M, N, O, P, Q, R, S, T, U, more than 20 derivative such as V, W etc. have been found and have isolated it to comprise Spinosyn A.
The commercialization kind has pleocidin Spinosyns (mixture of pleocidin A and pleocidin D) at present, the s-generation pleocidin insecticides Spinetoram. latter is got through semisynthesis by the thick product pleocidin L of biological method preparation and the mixture of J, promptly by 5 of pleocidin J, 6 two key selective reductions, reach 3 ‘ O-ethylization of rhamnosyl and obtain its major ingredient, ethylizing by 3 ‘ O-of pleocidin L rhamnosyl obtains its minor consistuent.
The pleocidin compound can be controlled lepidopteran, Diptera and Thysanoptera insect effectively.It can prevent and treat the pest species of some blade of eating in a large number in Coleoptera and the Orthoptera well.Pleocidin has very high activity to lepidopterous larvaes such as Heliothis virescens, bollworm, beet armyworm, prodenia litura, cabbage looper, small cabbage moth and rice-stem borers, and they are suitable environmental protection, have interesting toxicology character.
U.S. Patent No. 5362634 discloses the derivative that natural pleocidin is replaced by methyl or ethyl on C-21, U.S. Patent application No.60/153513 has disclosed the natural butenyl pleocidin derivative that the 3-4 carbochain replaces on C-21.Pleocidin derivative (John Daeuble, ThomasC.Sparks, Peter Johnson, Paul R.Graupner, the Bioorganic ﹠amp that can prepare C-21 position different substituents by replacement(metathesis)reaction; Medicinal Chemistry17 (2009) 4197-4205).U.S. Patent No. 6001981A, WO 9700265A have openly opened the chemosynthesis of pleocidin compound and have modified, and comprise aminosugar and rhamnosyl and the big chemically modified that encircles in the structure.
PATENT
https://patents.google.com/patent/WO2002077005A1/en
Spinosyns (A83543) are produced by derivatives of Saccharopolyspora spinosa NRRL18395 including strains NRRL 18537, 18538, 18539, 18719, 18720, 18743 and 18823 and derivatives thereof. A more preferred nomenclature for spinosyns is to refer to the pseudoaglycones as spinosyn A 17-Psa, spinosyn D 17-Psa, etc., and to the reverse pseudoaglycones as spinosyn A 9-Psa, spinosyn D 9-Psa, etc. (see Kirst et al., 1991). The known members of this family have been referred to as factors or components, and each has been given an identifying letter designation. These compounds are hereinafter referred to as spinosyn A, B, etc. The spinosyn compounds are useful for the control of arachnids, nematodes and insects, in particular Lepidoptera and Diptera species, and they are quite environmentally friendly and have an appealing toxicological profile. [0004] U.S. Patent No. 5,362,634 and corresponding European Patent Application No. 375316 Al disclose spinosyns A, B, C, D, E, F, G, H, and J. WO 93/09126 discloses spinosyns L, M, N, Q, R, S, and T. WO 94/20518 and US 5,6704,486 disclose spinosyns K,
O, P, U, V, W, and Y, and derivatives thereof. A large number of synthetic modifications to spinosyn compounds have been made, as disclosed in U.S. Patent No. 6,001,981 and WO
97/00265.
PAPER
J. Am. Chem. Soc. 120, 2553 (1998).
Further reading
- The non‐target impact of spinosyns on beneficial arthropods
- Spinosad toxicity to pollinators and associated risk
References
- ^ Jump up to:a b Mertz, Frederick; Raymond C. Yao (Jan 1990). “Saccharopolyspora spinosa sp. nov. Isolated from soil Collected in a Sugar Mill Rum Still”. International Journal of Systematic Bacteriology. 40 (1): 34–39. doi:10.1099/00207713-40-1-34.
- ^ Jump up to:a b c d e Qiao, Meihua; Daniel E. Snyder; Jeffery Meyer; Alan G. Zimmerman; Meihau Qiao; Sonya J. Gissendanner; Larry R. Cruthers; Robyn L. Slone; Davide R. Young (12 September 2007). “Preliminary Studies on the effectiveness of the novel pulicide, spinosad, for the treatment and control of fleas on dogs”. Veterinary Parasitology. 150 (4): 345–351. doi:10.1016/j.vetpar.2007.09.011. PMID 17980490.
- ^ Crouse, Gary; Thomas C Sparks; Joseph Schoonover; James Gifford; James Dripps; Tim Brue; Larry L Larson; Joseph Garlich; Chris Hatton; Rober L Hill; Thomas V Worden; Jacek G Martynow (27 September 2000). “Recent advances in the chemistry of spinosyns”. Pest Manag Sci. 57 (2): 177–185. doi:10.1002/1526-4998(200102)57:2<177::AID-PS281>3.0.CO;2-Z. PMID 11455648.
- ^ Jump up to:a b Watson, Gerald (31 May 2001). “Actions of Insecticidal Spinosyns on gama-Aminobutyric Acid Responses for Small-Diameter Cockroach Neurons”. Pesticide Biochemistry and Physiology. 71: 20–28. doi:10.1006/pest.2001.2559.
- ^ Jump up to:a b c d e Hertlein, Mark; Gary D. Thompson; Bhadriraju Subramanyam; Christos G. Athanassiou (12 January 2011). “Spinosad: A new natural product for stored grain protection”. Stored Products. 47 (3): 131–146. doi:10.1016/j.jspr.2011.01.004. Retrieved 3 May 2012.
- ^ Jump up to:a b Orr, Nailah; Andrew J. Shaffner; Kimberly Richey; Gary D. Crouse (30 April 2009). “Novel mode of action of spinosad: Receptor binding studies demonstrating lack of interaction with known insecticidal target sites”. Pesticide Biochemistry and Physiology. 95: 1–5. doi:10.1016/j.pestbp.2009.04.009.
- ^ Jump up to:a b c d Sparks, Thomas; Gary D crouse; Gregory Durst (30 March 2001). “Natural products as insecticides: the biology, biochemistry and quantitative structure-activity relationships of spinosyns and spinosoids”. Pest Manag Sci. 57 (10): 896–905. doi:10.1002/ps.358. PMID 11695182.
- ^ Jump up to:a b c Sparks, Thomas; James E. Dripps; Gerald B Watson; Doris Paroonagian (6 November 2012). “Resistance and cross-resistance to the spinosyns- A review and analysis”. Pesticide Biochemistry and Physiology. 102: 1–10. doi:10.1016/j.pestbp.2011.11.004. Retrieved 17 November 2011.
- ^ “Spinosad international brands”. Drugs.com. 3 January 2020. Retrieved 30 January2020.
- ^ “Spinosad US brands”. Drugs.com. 3 January 2020. Retrieved 30 January 2020.
- ^ “Spinosad – brand name list from”. Drugs.com. Retrieved 2012-10-20.
- ^ “UC Davis School of Vet Med”. Vetmed.ucdavis.edu. Retrieved 2012-10-20.
- ^ “Safer Flea Control | Insects in the City”. Citybugs.tamu.edu. Retrieved 2012-10-20.
- ^ “Codling Moth and Leafroller Control Using Chemicals” (PDF). Entomology.tfrec.wsu.edu. Retrieved 2012-10-20.
- ^ Stebbins, K. E. (2002). “Spinosad Insecticide: Subchronic and Chronic Toxicity and Lack of Carcinogenicity in CD-1 Mice”. Toxicological Sciences. 65 (2): 276–287. doi:10.1093/toxsci/65.2.276. PMID 11812932. Retrieved 2015-03-08.
- ^ Yano, B. L. (2002). “Spinosad Insecticide: Subchronic and Chronic Toxicity and Lack of Carcinogenicity in Fischer 344 Rats”. Toxicological Sciences. 65 (2): 288–298. doi:10.1093/toxsci/65.2.288. PMID 11812933. Retrieved 2015-03-08.
External links
- “Spinosad”. Drug Information Portal. U.S. National Library of Medicine.
- Monograph
| Spinosyn A | |
| Spinosyn D | |
| Identifiers | |
|---|---|
| CAS Number | 168316-95-8 (A)131929-60-7 (D) |
| ChEBI | CHEBI:9230 (A) CHEBI:9232 (D) |
| ChEMBL | ChEMBL1615373 |
| ChemSpider | 16736513 |
| ECHA InfoCard | 100.103.254 |
| PubChem CID | 183094 (A)443059 (D) |
| CompTox Dashboard (EPA) | DTXSID7032478 |
| InChI[show] | |
| Properties | |
| Chemical formula | C41H65NO10 (A) C42H67NO10 (D) |
| Pharmacology | |
| ATCvet code | QP53BX03 (WHO) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). |
//////////Spinosad
Dr. Darrin Lew https://www.drdarrinlew.us/insect-control/production-of-spinosad.html
Production of Spinosad
Last Updated on Tue, 29 Oct 2019 | Insect Control
Spinosad is produced directly from the fermentation of a strain of Saccharo-polyspora spinosa. Production strains of S. spinosa have been selected for increased titers of spinosyns A and D, however, no genetic engineering techniques have been used in this process and no genetically-modified organisms are used in the production process. After fermentation, the spinosyn A and D mixture is extracted from the fermentation broth, precipitated and dried to create technical spinosad, which is then formulated into end-use products. Spinosad technical material is also produced under pharmaceutical manufacturing guidelines to be used as a flea control agent in companion animals.
5.9.2 Production of Spinetoram
Production of spinetoram begins with the fermentation of a mutant strain of Saccharopolyspora spinosa that produces primarily spinosyns J and L, rather than spinosyns A and D. This strain was generated through mutagenesis of S. spinosa. However, like the spinosad-producing strains, no genetic engineering techniques were used in this process and no genetically-modified organisms are used in the production process. After fermentation, the spinosyn J and L mixture is extracted from the fermentation broth and precipitated in preparation for the two chemical synthesis steps required to produce spinetoram. The solvents used in extracting and precipitating the spinosyn J and L mixture are recycled.
Spinosyns J and L, unlike spinosyns A and D, have a free hydroxyl group at the 30-position on the rhamnose sugar, which allows for chemical manipulation of this site (see Figure 5.10). In the first synthetic step, the free hydroxyl at the 30-position in spinosyn J and spinosyn L is ethylated to yield a mixture of 30-O-ethyl spinosyn J and 30-O-ethyl spinosyn L. This material is then hydrogenated to yield a mixture of spinetoram-J (30-O-ethyl-5,6-dihydro spinosyn J; see Figure 5.2, structure 5.5) and spinetoram-L (30-O-ethyl spinosyn L; see Figure 5.2, structure 5.6). The hydrogenation conditions are selective and reduce only the disubstituted double bond between C5 and C6 in the 30-O-ethyl spinosyn J intermediate, leaving the 30-O-ethyl spinosyn L unchanged. The material is crystallized from the reaction mixture and dried to create technical spinetoram, which is then formulated into end-use products.
5.9.3 Formulation Attributes of the Spinosyns
To meet a variety of market needs, spinosad and spinetoram products span a very wide range of formulation types (see Table 5.8).
The range of possible formulations for any pesticide is determined by the physical and chemical properties of the active ingredient. Three primary properties determine the formulation characteristics of the spinosyns: (1) both Figure 5.10 Chemical synthesis steps in spinetoram manufacturing.
Figure 5.10 Chemical synthesis steps in spinetoram manufacturing.
Table 5.8 Spinosyn product formulation types and associated uses.
Formulation type
Use pattern
Suspension concentrate
Emulsifiable concentrate Wettable granule Wettable powder Dustable powder Sprayable bait Granular bait Bait stations Granules Tablets
Chewable tablets Gel, paste Creme rinse
Crops, ornamentals, forestry, stored grain, animal health, public health, turf, home and garden Public health Crops
Crops, ornamentals, seed treatment
Stored grain, crops
Crops
Crops, animal health, urban pests
Urban pests
Public health
Public health
Animal health
Urban pests
Public health are fermentation-derived mixtures; (2) both are weak bases; and (3) both have significant solubility in organic solvents.
As fermentation-derived products, spinosad and spinetoram are mixtures composed primarily of two similar, but not identical molecules. In terms of physical properties, a significant difference between the major and minor components of both spinosad and spinetoram is the presence or absence of a methyl group at C6 on the tetracycle (see Table 5.9). With regard to components of spinosad, spinosyn D (methyl group at C6) has a melting point 71 °C higher than that of spinosyn A (hydrogen at C6), and the water solubility of spinosyn D (at pH 7) is almost 1000-fold lower than that of spinosyn A. With regard to the components of spinetoram, spinetoram-L (methyl group at C6) has a melting point 72 °C lower than that of spinetoram-J (hydrogen at C6), and the water solubility of spinetoram-L (at pH 7) is four-fold higher than that of spinetoram-J. The melting points and water solubilities of the mixtures that constitute technical spinosad and technical spinetoram are determined by the relative ratios of the major and minor components.
The predominant components of both spinosad and spinetoram all have pKa values of about 8 (see Table 5.9). As a weak base, the solubility of spinosyns in water increases as the pH is reduced. From a formulation perspective, at pH level above 5, the spinosyns behave like high-melting solids with little water solubility, which results in the predominant agricultural formulations being suspension concentrates and wettable granule formulations composed of milled crystalline particles. Acid salts of spinosyns can be produced and are used in animal health formulations. The basic nature of the spinosyns is also a consideration when combining multiple active ingredients into the same formulation.
The spinosyns have significant solubility in organic solvents (see Table 5.9). This property is relatively rare in high-melting solids with limited water solubility, and has proven to be useful in a number of formulations for
Table 5.9 Selected physical properties of spinosyn A, spinosyn D, spinetoram-
J, and spinetoram-L.
Table 5.9 Selected physical properties of spinosyn A, spinosyn D, spinetoram-
J, and spinetoram-L.
| Property | Spinosyn A133 | Spinosyn D133 | Spinetoram-J134 | Spinetoram-L134 |
| Melting point, °C | 84-99.5a | 161.6-170a | 143.4b | 70.8b |
| Water solubility, | 235 | 0.332 | 11.3 | 46.7 |
| mg/lc’d’e | ||||
| pKaf | 8.10e | 7.87e | 7.86g | 7.59g |
| Solubility in organic solvents, mg/Lc | ||||
| Acetone | 168 000 | 10100 | >250000 | >250000 |
| Ethyl acetate | 194000 | 19 000 | >250000 | >250000 |
| w-Heptane | 12 400 | 300 | 23 900 | >250000 |
| Methanol | 190000 | 2520 | 163 000 | >250000 |
| Xylene | > 250 000 | 64000 | >250000 | >250000 |
“Visual determination. bDiffential scanning calorimetry. cShake flask. ^Buffered to pH 7. eAt 20 °C.
fCapillary zone electrophoresis. gAt 25 °C.
“Visual determination. bDiffential scanning calorimetry. cShake flask. ^Buffered to pH 7. eAt 20 °C.
fCapillary zone electrophoresis. gAt 25 °C.
non-agricultural markets, such as mosquito control and animal health. It is also a consideration when combining the spinosyns with other active ingredients.
////////
https://aem.asm.org/content/82/18/5603
