SILDENAFIL
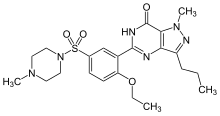
The chemical name of sildenafil is 5-[2-ethoxy-5-(4-methylpiperazin-1-ylsulfonyl)phenyl]-1- methyl-3-propyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one and its formula is C22H30N6O4S. The melting point of sildenafil is 189-190oC. Its solubility is 3.5 mg/mL in water.
The 1H NMR data of sildenafil is given below. The abbreviations used are s for singlet, d for doublet, t for triplet and q for quartet. The chemical shifts are given in ppm (parts per million) and are followed by the number of Hydrogens the peaks account for:
| peak (ppm) | integration | multiplicity |
| 0.94 | 3H | t |
| 1.32 | 3H | t |
| 2.15 | 3H | s |
| 2.35 | 4H | broad s |
| 2.76 | 2H | t |
| 2.88 | 4H | broad s |
| 4.14 | 3H | s |
| 4.18 | 2H | q |
| 7.36 | 1H | d |
| 7.80 | 2H | multiplet |
| 12.16 | 1H | broad s |
Sildenafil, sold as Viagra and other trade names, is a medication used to treat erectile dysfunction and pulmonary arterial hypertension.[1] Its effectiveness for treating sexual dysfunction in women has not been demonstrated.[1]
Common side effects include headaches and heart burn, as well as flushed skin. Caution is advised in those who have cardiovascular disease. Rare but serious side effects include prolonged erections, which can lead to damage to the penis, and sudden-onset hearing loss. Sildenafil should not be taken by people who take nitrates such as nitroglycerin, as this may result in a severe and potentially fatal drop in blood pressure.[1]
It acts by inhibiting cGMP-specific phosphodiesterase type 5 (PDE5), an enzyme that promotes degradation of cGMP, which regulates blood flow in the penis.
It was originally discovered by Pfizer scientists Andrew Bell, David Brown, and Nicholas Terrett.[2][3] Since becoming available in 1998, sildenafil has been a common treatment for erectile dysfunction; its primary competitors are tadalafil (Cialis) and vardenafil (Levitra).

EP0463756A,US6469012,WO2008074512A1
Chemical synthesis
Dunn PJ (2005). “Synthesis of Commercial Phosphodiesterase(V) Inhibitors”. Org Process Res Dev 2005 (1): 88–97. doi:10.1021/op040019c.
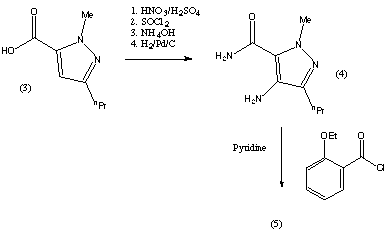
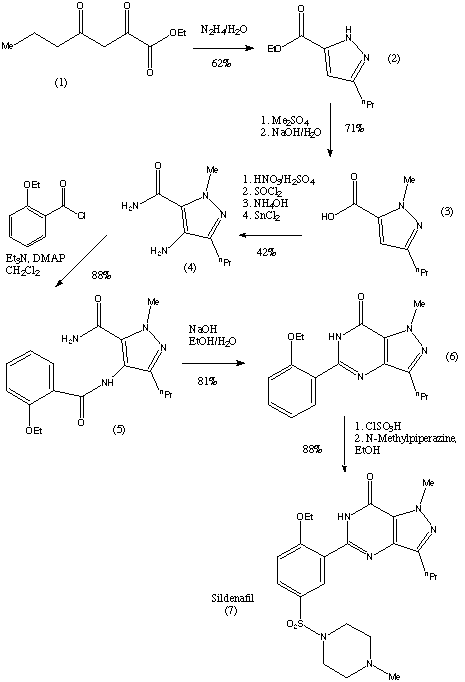
The preparation steps for synthesis of sildenafil are:[40]
- Methylation of 3-propylpyrazole-5-carboxylic acid ethyl ester with hot dimethyl sulfate
- Hydrolysis with aqueous NaOH to free acid
- Nitration with oleum/fuming nitric acid
- Carboxamide formation with refluxing thionyl chloride/NH4OH
- Reduction of nitro group to amino
- Acylation with 2-ethoxybenzoyl chloride
- Cyclization
- Sulfonation to the chlorosulfonyl derivative
- Condensation with 1-methylpiperazine.
|
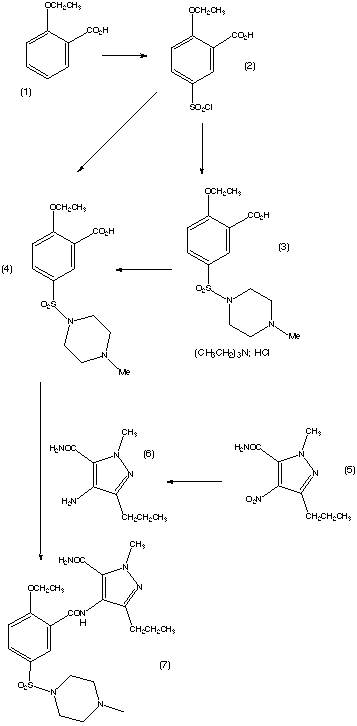
The synthesis of sildenafil citrate was first reported in the Bioorganic & Medicinal Chemistry Letters, Vol 6, pp. 1819, 1824, 1996. The reaction scheme is reproduced below. Sildenafil was reported in this journal as “a potent and selective inhibitor of type 5 PDE with utility for the treatment of male erectile dysfunction”. |
| he first step of the synthesis is the reaction of a diketoester (1) and hydrazine to give the pyrazole ring. The regioselective N-methylation of the pyrazole and hydrolysis gives a carboxylic acid (3). Compound (3) is then reacted with HNO3 and H2SO4 to give a nitrated product. This is then followed by a carboxamide formation and the reduction of the nitro group. The compound (4) is then acylated under basic conditions and this produces the pyrazolopyrimidinone (6). (6) is then chlorosulphonylated selectively on the 5′-position of the phenyl ring. This can then couple with an amine to give sildenafil (7). The yield of each step is given on the reaction scheme. |
|
This is the original synthesis which was reported in the literature when the molecule was first synthesised. A variant of the synthesis was published but the changes it involved only consisted in the change of a few reactants, and no major changes were reported. This synthesis appeared in the January 1999 issue of Chemistry in Britain. This journal only reported the original discovery synthesis and said that the synthesis used commercially had not been published. The drug is commercially manufactured by an alternative route. The reaction scheme is described in the patent which was published on 17 decembre 1997. However, the synthesis used in the commercial manufacture could be different to this. The patent was filed by the Pfizer Research and Development Company. The scheme is reproduced below.
The synthesis was described in a lot of detail, including the solvents that were the best to use, however, these details have not been reproduced here. These and further details about the synthesis can be found on the original patent document. The reaction pathway is explained in more detail below. The reagents employed in the reactions can vary, but the following are among the ones recommended by the submitters of the patent: |
|

SCHEME2

Chemical synthesis
…………………………………………………………………………………
SYNTHESIS

……………………………………………

…………………………………………………………………
SYNTHESIS

…………………………………………………………………………..

……………………………
PRECURSORS
…………………………………..
SYNTHESIS

Patents
European Union
Pfizer’s patent on sildenafil citrate expired in some member countries of the EU, Austria, Denmark, France, Germany, Ireland, Italy, The Netherlands, Spain, Sweden, the United Kingdom and Switzerland on 21 June 2013.[53][54][55] A UK patent held by Pfizer on the use of PDE5 inhibitors (see below) as treatment of impotence was invalidated in 2000 because of obviousness; this decision was upheld on appeal in 2002.[56][57]
United States
In 1992, Pfizer filed a patent covering the substance sildenafil and its use to treat cardiovascular diseases.[58] This patent was published in 1993 and expired in 2012. In 1994, Pfizer filed a patent covering the use of sildenafil to treat erectile dysfunction.[59] This patent was published in 2002 and will expire in 2019. Teva sued to have the latter patent invalidated, but Pfizer prevailed in an August 2011 federal district court case.[60]
The patent on Revatio (indicated for pulmonary arterial hypertension rather than erectile dysfunction) expired in late 2012. Generic versions of this low-dose form of sildenafil have been available in the U.S. from a number of manufacturers, including Greenstone, Mylan, and Watson, since early 2013.[61] No legal barrier exists to doctors prescribing this form of sildenafil “off label” for erectile dysfunction, although the dosage typically required for treating ED requires patients to take multiple pills.
Canada
In Canada, Pfizer’s patent 2,324,324 for Revatio (sildenafil used to treat pulmonary hypertension) was found invalid by the Federal Court in June 2010, on an application by Ratiopharm Inc.[62][63]
On November 8, 2012, the Supreme Court of Canada ruled that Pfizer’s patent 2,163,446 on Viagra was invalid from the beginning because the company did not provide full disclosure in its application. The decision, Teva Canada Ltd. v. Pfizer Canada Inc., pointed to section 27(3)(b) of The Patent Act which requires that disclosure must include sufficient information “to enable any person skilled in the art or science to which it pertains” to produce it. It added further: “As a matter of policy and sound statutory interpretation, patentees cannot be allowed to ‘game’ the system in this way. This, in my view, is the key issue in this appeal.”[64]
Teva Canada launched Novo-Sildenafil, a generic version of Viagra, on the day the Supreme Court of Canada released its decision.[65][66][67] To remain competitive, Pfizer then reduced the price of Viagra in Canada.[68] However, on November 9, 2012, Pfizer filed a motion for a re-hearing of the appeal in the Supreme Court of Canada,[69] on the grounds that the court accidentally exceeded its jurisdiction by voiding the patent.[70] Finally, on April 22, 2013, the Supreme Court of Canada invalidated Pfizer’s patent altogether.[71]
India
Manufacture and sale of sildenafil citrate drugs known as “generic viagra” is common in India, where Pfizer’s patent claim does not apply. Trade names include Kamagra (Ajanta Pharma), Silagra (Cipla), Edegra (Sun Pharmaceutical), Penegra (Zydus Cadila), and Zenegra (Alkem Laboratories).
China
Manufacture and sale of sildenafil citrate drugs is common in China, where Pfizer’s patent claim is not widely enforced.
Other countries
Egypt approved Viagra for sale in 2002, but soon afterwards allowed local companies to produce generic versions of the drug, citing the interests of poor people who would not be able to afford Pfizer’s price.[72]
Pfizer’s patent on sildenafil citrate expired in Brazil in 2010.[73]
References
- “Sildenafil Citrate”. The American Society of Health-System Pharmacists. Retrieved Dec 1, 2014.
- “Patent US5250534 – Pyrazolopyrimidinone antianginal agents – Google Patents”.
- Boolell M, Allen MJ, Ballard SA, Gepi-Attee S, Muirhead GJ, Naylor AM, Osterloh IH, Gingell C (June 1996). “Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction”. Int. J. Impot. Res. 8 (2): 47–52. PMID 8858389.
- Vardi M, Nini A (2007). Vardi, Moshe, ed. “Phosphodiesterase inhibitors for erectile dysfunction in patients with diabetes mellitus”. Cochrane Database Syst Rev (1): CD002187. doi:10.1002/14651858.CD002187.pub3. PMID 17253475.
- “FDA Approves Pfizer’s Revatio as Treatment for Pulmonary Arterial Hypertension” (Press release). Pfizer. June 6, 2005. Archived from the original on August 28, 2005.
- Nurnberg HG, Hensley PL, Gelenberg AJ, Fava M, Lauriello J, Paine S (January 2003). “Treatment of antidepressant-associated sexual dysfunction with sildenafil: a randomized controlled trial”. JAMA 289 (1): 56–64. doi:10.1001/jama.289.1.56. PMID 12503977.
- Nurnberg HG, Hensley PL, Lauriello J, Parker LM, Keith SJ (August 1999). “Sildenafil for women patients with antidepressant-induced sexual dysfunction”. Psychiatr Serv 50 (8): 1076–8. PMID 10445658.
- Nurnberg HG, Hensley PL, Heiman JR, Croft HA, Debattista C, Paine S (2008). “Sildenafil Treatment of Women With Antidepressant-Associated Sexual Dysfunction”. JAMA 300 (4): 395–404. doi:10.1001/jama.300.4.395. PMID 18647982.
- Richalet JP, Gratadour P, Robach P, et al. (2005). “Sildenafil inhibits altitude-induced hypoxemia and pulmonary hypertension”. Am. J. Respir. Crit. Care Med. 171 (3): 275–81. doi:10.1164/rccm.200406-804OC. PMID 15516532.
- Perimenis P (2005). “Sildenafil for the treatment of altitude-induced hypoxaemia”. Expert Opin Pharmacother 6 (5): 835–7. doi:10.1517/14656566.6.5.835. PMID 15934909.
- Fagenholz PJ, Gutman JA, Murray AF, Harris NS (2007). “Treatment of high altitude pulmonary edema at 4240 m in Nepal”. High Alt. Med. Biol. 8 (2): 139–46. doi:10.1089/ham.2007.3055. PMID 17584008.
- “Viagra Prescribing Information”. Pfizer. October 2007. Archived from the original (PDF) on 14 November 2012. Retrieved 14 November 2012.
- “Viagra and vision”. VisionWeb. 29 October 2001. Retrieved 2009-02-10.
- “FDA Updates Labeling for Viagra, Cialis and Levitra for Rare Post-Marketing Reports of Eye Problems”. United States Food and Drug Administration. 8 July 2005. Archived from the original on February 23, 2008. Retrieved 2009-02-10.
- Laties AM (2009). “Vision disorders and phosphodiesterase type 5 inhibitors: a review of the evidence to date”. Drug Saf 32 (1): 1–18. doi:10.2165/00002018-200932010-00001. PMID 19132801.
- “FDA Announces Revisions to Labels for Cialis, Levitra and Viagra”. United States Food and Drug Administration. 18 October 2007. Archived from the original on 11 July 2007. Retrieved 10 February 2009.
- “Viagra (sildenafil citrate) tablets” (PDF). page 29: Pzifer. October 2007. Retrieved 2009-10-25.
- Kloner RA (2005). “Pharmacology and drug interaction effects of the phosphodiesterase 5 inhibitors: focus on alpha-blocker interactions”. Am J Cardiol 96 (12B): 42M–46M. doi:10.1016/j.amjcard.2005.07.011. PMID 16387566.
- Cheitlin MD, Hutter AM Jr, Brindis RG, Ganz P, Kaul S, Russell RO Jr, Zusman RM (1999). “ACC/AHA expert consensus document. Use of sildenafil (Viagra) in patients with cardiovascular disease. American College of Cardiology/American Heart Association”. Journal of the American College of Cardiology 33 (1): 273–82. doi:10.1016/S0735-1097(98)00656-1. PMID 9935041.
- Peterson K (2001-03-21). “Young men add Viagra to their drug arsenal”. USAToday.
- Smith KM, Romanelli F (2005). “Recreational use and misuse of phosphodiesterase 5 inhibitors”. J Am Pharm Assoc (2003) 45 (1): 63–72; quiz 73–5. doi:10.1331/1544345052843165. PMID 15730119.
- Sildenafil Will Not Affect Libido – Fact!
- Mondaini N, Ponchietti R, Muir GH, Montorsi F, Di Loro F, Lombardi G, Rizzo M (June 2003). “Sildenafil does not improve sexual function in men without erectile dysfunction but does reduce the postorgasmic refractory time”. Int. J. Impot. Res. 15 (3): 225–8. doi:10.1038/sj.ijir.3901005. PMID 12904810.
- McCambridge J, Mitcheson L, Hunt N, Winstock A (March 2006). “The rise of Viagra among British illicit drug users: 5-year survey data”. Drug Alcohol Rev 25 (2): 111–3. doi:10.1080/09595230500537167. PMID 16627299.
- Eloi-Stiven ML, Channaveeraiah N, Christos PJ, Finkel M, Reddy R (November 2007). “Does marijuana use play a role in the recreational use of sildenafil?”. J Fam Pract 56 (11): E1–4. PMID 17976333.
- “The 2007 Ig Nobel Prize Winners”. Improbable Research. 4 October 2007. Retrieved 2009-02-10.
- Agostino PV, Plano SA, Golombek DA (June 2007). “Sildenafil accelerates reentrainment of circadian rhythms after advancing light schedules”. Proc. Natl. Acad. Sci. U.S.A. 104 (23): 9834–9. doi:10.1073/pnas.0703388104. PMC 1887561. PMID 17519328.
- Teri Thompson, Christian Red, Michael O’Keefffe, and Nathaniel Vinton (10 June 2008). “Source: Roger Clemens, host of athletes pop Viagra to help onfield performance”. Daily News (Daily News). Retrieved 2009-02-10.
- Busbee J (2012-11-28). “Bears’ Brandon Marshall says some NFL players use Viagra … ON THE FIELD”. Yahoo! Sports. Retrieved 2012-11-28.
- Venhuis BJ, de Kaste D. (2006–2012). “Towards a decade of detecting new analogues of sildenafil, tadalafil and vardenafil in food supplements: a history, analytical aspects and health risks”. Journal of Pharmaceutical and Biomedical Analysis 69: 196–208. doi:10.1016/j.jpba.2012.02.014. PMID 22464558.
- Oh SS, Zou P, Low MY, Koh HL. Detection of sildenafil analogues in herbal products for erectile dysfunction (2006). “Detection of sildenafil analogues in herbal products for erectile dysfunction”. Journal of Toxicology and Environmental Health Part A 69 (21): 1951–1958. doi:10.1080/15287390600751355. PMID 16982533.
- Venhuis BJ, Blok-Tip L, de Kaste D (2008). “Designer drugs in herbal aphrodisiacs”. Forensic Science International 177 (2–3): 25–27. doi:10.1016/j.forsciint.2007.11.007. PMID 18178354.
- FDA letter to Libidus distributor
- FDA Warns Consumers About Dangerous Ingredients in “Dietary Supplements” Promoted for Sexual Enhancement
- Hidden Risks of Erectile Dysfunction “Treatments” Sold Online
- R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 9th edition, Biomedical Publications, Seal Beach, CA, 2011, pp. 1552–1553. http://www.biomedicalpublications.com/dt9.pdf
- Sung, B. J.; Hwang, K.; Jeon, Y.; Lee, J. I.; Heo, Y. S.; Kim, J.; Moon, J.; Yoon, J.; Hyun, Y. L.; Kim, E.; Eum, S.; Park, S. Y.; Lee, J. O.; Lee, T.; Ro, S.; Cho, J. (2003). “Structure of the catalytic domain of human phosphodiesterase 5 with bound drug molecules”. Nature 425 (6953): 98–102. doi:10.1038/nature01914. PMID 12955149.
- Webb, D.J.; Freestone, S.; Allen, M.J.; Muirhead, G.J. (March 4, 1999). “Sildenafil citrate and blood-pressure-lowering drugs: results of drug interaction studies with an organic nitrate and a calcium antagonist”. Am. J. Cardiol. 83 (5A): 21C–28C. doi:10.1016/S0002-9149(99)00044-2. PMID 10078539.
- “Viagra Clinical Pharmacology”. RxList.com. 2008. Retrieved 2008-08-20.
- Dunn PJ (2005). “Synthesis of Commercial Phosphodiesterase(V) Inhibitors”. Org Process Res Dev 2005 (1): 88–97. doi:10.1021/op040019c.
- “Research”. ABM. Abertawe Bro Morgannwg University Health Board. 4 July 2008. Archived from the original on 26 September 2008. Retrieved 6 August 2008.
Our clinicians regularly offer patients the opportunity to take part in trials of new drugs and treatments. Morriston Hospital in Swansea, was the first in the world to trial Viagra!
- Terrett NK, Bell AS, Brown D, Elllis P (1996). “Sildenafil (Viagra), a potent and selective inhibitor of Type 5 cGMP phosphodiesterase with utility for the treatment of male erectile dysfunction”. Bioorg Med Chem Lett 6 (15): 1819–1824. doi:10.1016/0960-894X(96)00323-X.
- Kling J (1998). “From hypertension to angina to Viagra”. Mod. Drug Discov. 1: 31–38. ISSN 1532-4486. OCLC 41105083.
- Viagra sales peak at $1,934m in 2008
- Ciment, J (1999). “Missouri fines internet pharmacy”. BMJ (British Medical Journal) 319 (7221): 1324. doi:10.1136/bmj.319.7221.1324g. PMC 1174637. PMID 10567131.
- Devine, Amy (September 29, 2008). “Chemists plan to sell Viagra on the internet”. Daily Record. Retrieved 2012-04-30.
- Keith A (2000). “The economics of Viagra”. Health Aff (Millwood) 19 (2): 147–57. doi:10.1377/hlthaff.19.2.147. PMID 10718028.
- McGuire S (2007-01-01). “Cialis gaining market share worldwide”. Medical Marketing & Media. Haymarket Media. Archived from the original on 2009-01-03. Retrieved 2009-02-10.
- Mullin, Rick (June 20, 2005). “Viagra”. Chemical & Engineering News 83 (25). Retrieved 2008-08-20.
- Berenson, Alex (December 4, 2005). “Sales of Impotence Drugs Fall, Defying Expectations”. The New York Times. Retrieved 2008-08-20.
- “Over-the-counter Viagra piloted”. BBC News. BBC News. 11 February 2007. Retrieved 2009-02-10.
- “Pfizer to sell Viagra online, in first for Big Pharma: AP”. CBS News. Retrieved 6 May 2013.
- “Actavis Launches Generic Viagra in Europe as Patents Expire”. Retrieved 2013-10-25.
- Jim Edwards (October 21, 2009). “What Will Happen When Viagra Goes Generic?”. AccessRx.com. Retrieved 2011-03-25.
- “Is Viagra about to lose its pulling power in the UK?”. The Guardian. Retrieved 13 June 2013.
- Murray-, Rosie (23 January 2002). “Viagra ruling upsets Pfizer”. London: Telegraph Media Group Limited. Archived from the original on 22 August 2009. Retrieved 10 February 2009.
- “Pfizer Loses UK Battle on Viagra Patent”. UroToday. Thomson Reuters. 17 June 2002. Archived from the original on 25 June 2007. Retrieved 10 February 2009.
- U.S. Patent 5,250,534
- U.S. Patent 6,469,012
- “Pfizer Wins Viagra Patent Infringement Case Against Teva Pharmaceuticals”. Bloomberg. August 15, 2011. Retrieved 2012-04-01.
- “Pfizer’s Revatio Goes Generic”. Zacks Equity Research. November 15, 2012. Retrieved 2013-10-05.
- “Revation patent ruled invalid for lack of sound prediction and obviousness”. Canadian Technology & IP Law. Stikeman Elliott. 2010-06-18. Retrieved 2012-11-14.
- “Pfizer Canada Inc. v. Ratiopharm Inc., 2010 FC 612″. CanLII.
- Teva Canada Ltd. v. Pfizer Canada Inc. 2012 SCC 60 at par. 80 (8 November 2012)
- John Spears (2012-11-08). “Supreme Court ruling could lead to cheaper versions of Viagra”. The [Toronto] Star. Retrieved 2012-11-14.
- Ken Hanly (2012-11-08). “Canadian Supreme court rules Viagra patent invalid”. Digital Journal. Retrieved 2012-11-14.
- “Viagra patent tossed out by Supreme Court: Decision allows generic versions of drug to be produced”. CBC News. 2012-11-08. Retrieved 2012-11-14.
- “Pfizer Canada drops Viagra price after generic versions get Supreme Court green light”. Financial Post. 2012-11-22. Retrieved 2013-02-09.
- “SCC Case Information, Docket No. 33951”. Retrieved 2012-11-14.
- Kirk Makin (2012-11-15). “In rare move, Pfizer asks Supreme Court to reconsider ruling that killed Viagra patent”. The Globe and Mail. Retrieved 2012-11-15.
- Gowling Lafleur Henderson LLP, Hélène D’Iorio (2013-04-22). “The Supreme Court of Canada holds Pfizer’s Viagra patent invalid”. Lexology. Retrieved 2013-12-27.
- Allam, Abeer (October 4, 2002). “Seeking Investment, Egypt Tries Patent Laws”. New York Times. Retrieved April 1, 2013.
External link
Official Viagra Website
- Official Viagra UK Website
- Official Revatio Website
- prescribing information for Viagra and prescribing information for Revatio from Pfizer
- FDA Information
- MedlinePLUS information, including side effects
- U.S. National Library of Medicine: Drug Information Portal – Sildenafil
- Viagra at The Periodic Table of Videos (University of Nottingham)
 |
|
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
1-[4-ethoxy-3-(6,7-dihydro-1-methyl-
7-oxo-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-5-yl) phenylsulfonyl]-4-methylpiperazine |
|
| Clinical data | |
| Trade names | Viagra, Revatio, others |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a699015 |
| Licence data | EMA:Link, US FDA:link |
| Pregnancy category |
|
| Legal status |
|
| Routes of administration |
Oral, IV |
| Pharmacokinetic data | |
| Bioavailability | 40% |
| Metabolism | Hepatic (mostly CYP3A4, also CYP2C9) |
| Biological half-life | 3 to 4 hours |
| Excretion | Fecal (80%) and renal (around 13%) |
| Identifiers | |
| CAS Registry Number | 139755-83-2  |
| ATC code | G04BE03 |
| PubChem | CID: 5281023 |
| DrugBank | DB00203  |
| ChemSpider | 56586  |
| UNII | 3M7OB98Y7H  |
| KEGG | D08514  |
| ChEBI | CHEBI:58987  |
| ChEMBL | CHEMBL1737  |
| PDB ligand ID | VIA (PDBe, RCSB PDB) |
| Chemical data | |
| Formula | C22H30N6O4S |
| Molecular mass | base: 474.6 g/mol |
///////
Filed under: Uncategorized Tagged: SILDENAFIL