

TERIPARATIDE
テリパラチド;
- PTH 1-34
- LY 333334 / LY-333334 / LY333334 / ZT-034
|
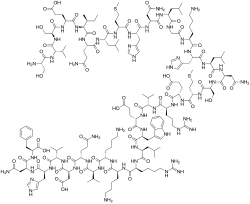
Ser Val Ser Glu Ile Gln Leu Met His Asn Leu Gly Lys His Leu Asn
Ser Met Glu Arg Val Glu Trp Leu Arg Lys Lys Leu Gln Asp Val His Asn Phe-OH |
|
| Type |
Peptide
|
|---|
| Formula |
C181H291N55O51S2
|
|---|---|
| CAS |
52232-67-4
99294-94-7 (acetate)
|
| Mol weight |
4117.7151
|
(4S)-4-[[(2S)-2-[[(2S)-2-[[(2S)-4-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-6-amino-2-[[2-[[(2S)-2-[[(2S)-4-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-5-amino-2-[[(2S,3S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-amino-3-hydroxypropanoyl]amino]-3-methylbutanoyl]amino]-3-hydroxypropanoyl]amino]-4-carboxybutanoyl]amino]-3-methylpentanoyl]amino]-5-oxopentanoyl]amino]-4-methylpentanoyl]amino]-4-methylsulfanylbutanoyl]amino]-3-(1H-imidazol-5-yl)propanoyl]amino]-4-oxobutanoyl]amino]-4-methylpentanoyl]amino]acetyl]amino]hexanoyl]amino]-3-(1H-imidazol-5-yl)propanoyl]amino]-4-methylpentanoyl]amino]-4-oxobutanoyl]amino]-3-hydroxypropanoyl]amino]-4-methylsulfanylbutanoyl]amino]-5-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[(2S)-6-amino-1-[[(2S)-1-[[(2S)-5-amino-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-4-amino-1-[[(1S)-1-carboxy-2-phenylethyl]amino]-1,4-dioxobutan-2-yl]amino]-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-3-carboxy-1-oxopropan-2-yl]amino]-1,5-dioxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-1-oxohexan-2-yl]amino]-1-oxohexan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-5-oxopentanoic acid
| SVG Image | |
|---|---|
| IUPAC Condensed | H-Ser-Val-Ser-Glu-Ile-Gln-Leu-Met-His-Asn-Leu-Gly-Lys-His-Leu-Asn-Ser-Met-Glu-Arg-Val-Glu-Trp-Leu-Arg-Lys-Lys-Leu-Gln-Asp-Val-His-Asn-Phe-OH |
| Sequence | SVSEIQLMHNLGKHLNSMERVEWLRKKLQDVHNF |
| PLN | H-SVSEIQLMHNLGKHLNSMERVEWLRKKLQDVHNF-OH |
| HELM | PEPTIDE1{S.V.S.E.I.Q.L.M.H.N.L.G.K.H.L.N.S.M.E.R.V.E.W.L.R.K.K.L.Q.D.V.H.N.F}$$$$ |
| IUPAC | L-seryl-L-valyl-L-seryl-L-alpha-glutamyl-L-isoleucyl-L-glutaminyl-L-leucyl-L-methionyl-L-histidyl-L-asparagyl-L-leucyl-glycyl-L-lysyl-L-histidyl-L-leucyl-L-asparagyl-L-seryl-L-methionyl-L-alpha-glutamyl-L-arginyl-L-valyl-L-alpha-glutamyl-L-tryptophyl-L-leucyl-L-arginyl-L-lysyl-L-lysyl-L-leucyl-L-glutaminyl-L-alpha-aspartyl-L-valyl-L-histidyl-L-asparagyl-L-phenylalanine |
Other Names
- L-Seryl-L-valyl-L-seryl-L-α-glutamyl-L-isoleucyl-L-glutaminyl-L-leucyl-L-methionyl-L-histidyl-L-asparaginyl-L-leucylglycyl-L-lysyl-L-histidyl-L-leucyl-L-asparaginyl-L-seryl-L-methionyl-L-α-glutamyl-L-arginyl-L-valyl-L-α-glutamyl-L-tryptophyl-L-leucyl-L-arginyl-L-lysyl-L-lysyl-L-leucyl-L-glutaminyl-L-α-aspartyl-L-valyl-L-histidyl-L-asparaginyl-L-phenylalanine
- (1-34)-Human parathormone
- (1-34)-Human parathyroid hormone
- 1-34-Human PTH
- 1-34-Parathormone (human)
- 11: PN: WO0039278 SEQID: 17 unclaimed protein
- 14: PN: WO0181415 SEQID: 16 claimed protein
- 15: PN: WO0123521 SEQID: 19 claimed protein
- 1: PN: EP2905289 SEQID: 1 claimed protein
- 1: PN: WO0198348 SEQID: 13 claimed protein
- 1: PN: WO2011071480 SEQID: 14 claimed protein
- 225: PN: US20090175821 SEQID: 272 claimed protein
- 22: PN: US6110892 SEQID: 22 unclaimed protein
- 2: PN: US20100261199 SEQID: 4 claimed protein
- 31: PN: US20070099831 PAGE: 7 claimed protein
- 32: PN: WO2008068487 SEQID: 32 claimed protein
- 5: PN: WO2008033473 SEQID: 4 claimed protein
- 692: PN: WO2004005342 PAGE: 46 claimed protein
- 69: PN: US20050009742 PAGE: 20 claimed sequence
- 7: PN: WO0031137 SEQID: 8 unclaimed protein
- 7: PN: WO0040611 PAGE: 1 claimed protein
- 93: PN: WO0069900 SEQID: 272 unclaimed protein
- Forsteo
- Forteo
- HPTH-(1-34)
- Human PTH(1-34)
- Human parathormone(1-34)
- Human parathyroid hormone-(1-34)
- LY 333334
- Osteotide
- Parathar
- Parathormone (human)
- Teriparatide
- ZT 034
Product Ingredients
| INGREDIENT | UNII | CAS | |
|---|---|---|---|
| Teriparatide acetate | 9959P4V12N | 99294-94-7 |
Teriparatide is a form of parathyroid hormone consisting of the first (N-terminus) 34 amino acids, which is the bioactive portion of the hormone. It is an effective anabolic (promoting bone formation) agent[2] used in the treatment of some forms of osteoporosis.[3] It is also occasionally used off-label to speed fracture healing. Teriparatide is identical to a portion of human parathyroid hormone (PTH) and intermittent use activates osteoblasts more than osteoclasts, which leads to an overall increase in bone.
Recombinant teriparatide is sold by Eli Lilly and Company under the brand name Forteo/Forsteo. A synthetic teriparatide from Teva Generics has been authorised for marketing in European territories[4]. Biosimilar product from Gedeon Richter plc has been authorised in Europe[5]. On October 4, 2019 the US FDA approved a recombinant teriparatide product, PF708, from Pfenex Inc. PF708 is the first FDA approved proposed therapeutic equivalent candidate to Forteo.
Teriparatide (recombinant human parathyroid hormone) is a potent anabolic agent used in the treatment of osteoporosis. It is manufactured and marketed by Eli Lilly and Company.
Teriparatide is a recombinant form of parathyroid hormone. It is an effective anabolic (i.e., bone growing) agent used in the treatment of some forms of osteoporosis. It is also occasionally used off-label to speed fracture healing. Teriparatide is identical to a portion of human parathyroid hormone (PTH) and intermittent use activates osteoblasts more than osteoclasts, which leads to an overall increase in bone. Teriparatide is sold by Eli Lilly and Company under the brand name Forteo.
Indication
For the treatment of osteoporosis in men and postmenopausal women who are at high risk for having a fracture. Also used to increase bone mass in men with primary or hypogonadal osteoporosis who are at high risk for fracture.
Associated Conditions
Pharmacodynamics
Clinical trials indicate that teriparatide increases predominantly trabecular bone in the lumbar spine and femoral neck; it has less significant effects at cortical sites. The combination of teriparatide with antiresorptive agents is not more effective than teriparatide monotherapy. The most common adverse effects associated with teriparatide include injection-site pain, nausea, headaches, leg cramps, and dizziness. After a maximum of two years of teriparatide therapy, the drug should be discontinued and antiresorptive therapy begun to maintain bone mineral density.
Mechanism of action
Teriparatide is the portion of human parathyroid hormone (PTH), amino acid sequence 1 through 34 of the complete molecule which contains amino acid sequence 1 to 84. Endogenous PTH is the primary regulator of calcium and phosphate metabolism in bone and kidney. Daily injections of teriparatide stimulates new bone formation leading to increased bone mineral density.
Medical uses
Teriparatide has been FDA-approved since 2002.[6] It is effective in growing bone (e.g., 8% increase in bone density in the spine after one year)[7] and reducing the risk of fragility fractures.[6][8] When studied, teriparatide only showed bone mineral density (BMD) improvement during the first 18 months of use. Teriparatide should only be used for a period of 2 years maximum. After 2 years, another agent such a bisphosphonate or denosumab should be used in cases of osteoporosis. [9]
Teriparatide cuts the risk of hip fracture by more than half but does not reduce the risk of arm or wrist fracture.[10]
Other
Teriparatide can be used off-label to speed fracture repair and treat fracture nonunions.[11] It has been reported to have been successfully used to heal fracture nonunions.[12] Generally, due to HIPAA regulations, it is not publicized when American athletes receive this treatment to improve fracture recovery.[11] But an Italian football player, Francesco Totti, was given teriparatide after a tibia/fibula fracture, and he unexpectedly recovered in time for the 2006 World Cup.[11] It has been reported that Mark Mulder used it to recover from a hip fracture Oakland A’s for the 2003 MLB playoffs[13] and Terrell Owens to recover from an ankle fracture before the 2005 Super Bowl.[13]
Administration
Teriparatide is administered by injection once a day in the thigh or abdomen.
Contraindications
Teriparatide should not be prescribed for people who are at increased risks for osteosarcoma. This includes those with Paget’s Diseaseof bone or unexplained elevations of serum alkaline phosphate, open epiphysis, or prior radiation therapy involving the skeleton. In the animal studies and in one human case report, it was found to potentially be associated with developing osteosarcoma in test subjects after over 2 years of use. [14]
Patients should not start teriparatide until any vitamin D deficiency is corrected. [15]
Adverse effects
Adverse effects of teriparatide include headache, nausea, dizziness, and limb pain.[6] Teriparatide has a theoretical risk of osteosarcoma, which was found in rat studies but not confirmed in humans.[2] This may be because unlike humans, rat bones grow for their entire life.[2] The tumors found in the rat studies were located on the end of the bones which grew after the injections began.[15]After nine years on the market, there were only two cases of osteosarcoma reported.[7] This risk was considered by the FDA as “extremely rare” (1 in 100,000 people)[6] and is only slightly more than the incidence in the population over 60 years old (0.4 in 100,000).[6]
Mechanism of action
Teriparatide is a portion of human parathyroid hormone (PTH), amino acid sequence 1 through 34, of the complete molecule (containing 84 amino acids). Endogenous PTH is the primary regulator of calcium and phosphate metabolism in bone and kidney. PTH increases serum calcium, partially accomplishing this by increasing bone resorption. Thus, chronically elevated PTH will deplete bone stores. However, intermittent exposure to PTH will activate osteoblasts more than osteoclasts. Thus, once-daily injections of teriparatide have a net effect of stimulating new bone formation leading to increased bone mineral density.[16][17][18]
Teriparatide is the first FDA approved agent for the treatment of osteoporosis that stimulates new bone formation.[19]
FDA approval
Teriparatide was approved by the Food and Drug Administration (FDA) on 26 November 2002, for the treatment of osteoporosis in men and postmenopausal women who are at high risk for having a fracture. The drug is also approved to increase bone mass in men with primary or hypogonadal osteoporosis who are at high risk for fracture.
Combined teriparatide and denosumab
Combined teriparatide and denosumab increased BMD more than either agent alone and more than has been reported with approved therapies. Combination treatment might, therefore, be useful to treat patients at high risk of fracture by increasing BMD. However, there is no evidence of fracture rate reduction in patients taking a teriparatide and denosumab combination. Moreover, the combination therapy group showed a significant decrease in their bone formation marker, indicating that denosumab, an antiresorptive agent, might actually counteract the effect of teriparatide, a bone formation anabolic agent, in bone formation. [20]
PATENT
KR 2011291
WO 2019077432
CN 109897099
CN 109879955
CN 109879954
CN 108373499
PATENT
WO-2020000555
Process for preparing teriparatide as parathyroid hormone receptor agonist, useful for treating osteoporosis in menopausal women. Appears to be the first filing from the assignee and the inventors on this compound, however, this invention was previously seen as a Chinese national filing published in 12/2013. Daiichi Sankyo , through its subsidiary Asubio Pharma , was developing SUN-E-3001 , a nasally administered recombinant human parathyroid hormone, for the treatment of osteoporosis.
Teriparatide is a 1-34 fragment of human parathyroid hormone, which has the same biological activity as human parathyroid hormone. Hypogonadous osteoporosis and osteoporosis in menopausal women have great market prospects.
In patent CN201410262511, a pseudoproline dipeptide Fmoc-Asn (Trt) -Ser (ψ Me, Me Pro) -OH is used instead of the two amino acids at the original 16-17 positions for coupling one by one, and the final cleavage yields teriparatide. This method adopts the method of feeding pseudoproline dipeptide to avoid the generation of oxidative impurities, but it cannot avoid a variety of missing peptides due to the excessively long peptide chain. At the same time, the pseudoproline dipeptide is expensive and difficult to obtain.
References
- ^ http://www.minsa.gob.pa/sites/default/files/alertas/nota_seguridad_teriparatida.pdf
- ^ Jump up to:a b c Riek AE and Towler DA (2011). “The pharmacological management of osteoporosis”. Missouri Medicine. 108 (2): 118–23. PMC 3597219. PMID 21568234.
- ^ Saag KG, Shane E, Boonen S, et al. (November 2007). “Teriparatide or alendronate in glucocorticoid-induced osteoporosis”. The New England Journal of Medicine. 357 (20): 2028–39. doi:10.1056/NEJMoa071408. PMID 18003959.
- ^ BfArM (2017-05-08). “PUBLIC ASSESSMENT REPORT – Decentralised Procedure – Teriparatid-ratiopharm 20 µg / 80ml, Solution for injection” (PDF).
- ^ “Summary of the European public assessment report (EPAR) for Terrosa”. Retrieved 2019-08-14.
- ^ Jump up to:a b c d e Rizzoli, R.; Reginster, J. Y.; Boonen, S.; Bréart, G. R.; Diez-Perez, A.; Felsenberg, D.; Kaufman, J. M.; Kanis, J. A.; Cooper, C. (2011). “Adverse Reactions and Drug–Drug Interactions in the Management of Women with Postmenopausal Osteoporosis”. Calcified Tissue International. 89 (2): 91–104. doi:10.1007/s00223-011-9499-8. PMC 3135835. PMID 21637997.
- ^ Jump up to:a b Kawai, M.; Mödder, U. I.; Khosla, S.; Rosen, C. J. (2011). “Emerging therapeutic opportunities for skeletal restoration”. Nature Reviews Drug Discovery. 10 (2): 141–156. doi:10.1038/nrd3299. PMC 3135105. PMID 21283108.
- ^ Murad, M. H.; Drake, M. T.; Mullan, R. J.; Mauck, K. F.; Stuart, L. M.; Lane, M. A.; Abu Elnour, N. O.; Erwin, P. J.; Hazem, A.; Puhan, M. A.; Li, T.; Montori, V. M. (2012). “Comparative Effectiveness of Drug Treatments to Prevent Fragility Fractures: A Systematic Review and Network Meta-Analysis”. Journal of Clinical Endocrinology & Metabolism. 97(6): 1871–1880. doi:10.1210/jc.2011-3060. PMID 22466336.
- ^ O’Connor KM. Evaluation and Treatment of Osteoporosis. Med Clin N Am. 2016; 100:807-26
- ^ Díez-Pérez A, Marin F, Eriksen EF, Kendler DL, Krege JH, Delgado-Rodríguez M (September 2018). “Effects of teriparatide on hip and upper limb fractures in patients with osteoporosis: A systematic review and meta-analysis”. Bone. 120: 1–8. doi:10.1016/j.bone.2018.09.020. PMID 30268814.
- ^ Jump up to:a b c Bruce Jancin (2011-12-12). “Accelerating Fracture Healing With Teriparatide”. Internal Medicine News Digital Network. Retrieved 2013-09-20.
- ^ Giannotti, S.; Bottai, V.; Dell’Osso, G.; Pini, E.; De Paola, G.; Bugelli, G.; Guido, G. (2013). “Current medical treatment strategies concerning fracture healing”. Clinical Cases in Mineral and Bone Metabolism. 10 (2): 116–120. PMC 3796998. PMID 24133528.
- ^ Jump up to:a b William L. Carroll (2005). “Chapter 1: Defining the Issue”. The Juice: The Real Story of Baseball’s Drug Problems. ISBN 1-56663-668-X. Retrieved 2013-09-23.
- ^ Harper KD, Krege JH, Marcus R, et al. Osteosarcoma and teriparatide? J Bone Miner Res 2007;22(2):334
- ^ Jump up to:a b https://www.drugs.com/pro/forteo.html
- ^ Bauer, E; Aub, JC; Albright, F (1929). “Studies of calcium and phosphorus metabolism: V. Study of the bone trabeculae as a readily available reserve supply of calcium”. J Exp Med. 49 (1): 145–162. doi:10.1084/jem.49.1.145. PMC 2131520. PMID 19869533.
- ^ Selye, H (1932). “On the stimulation of new bone formation with parathyroid extract and irradiated ergosterol”. Endocrinology. 16 (5): 547–558. doi:10.1210/endo-16-5-547.
- ^ Dempster, D. W.; Cosman, F.; Parisien, M.; Shen, V.; Lindsay, R. (1993). “Anabolic actions of parathyroid hormone on bone”. Endocrine Reviews. 14 (6): 690–709. doi:10.1210/edrv-14-6-690. PMID 8119233.
- ^ Fortéo: teriparatide (rDNA origin) injection Archived 2009-12-27 at the Wayback Machine
- ^ Tsai, Joy N; Uihlein, Alexander V; Lee, Hang; Kumbhani, Ruchit; Siwila-Sackman, Erica; McKay, Elizabeth A; Burnett-Bowie, Sherri-Ann M; Neer, Robert M; Leder, Benjamin Z (2013). “Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: The DATA study randomised trial”. The Lancet. 382 (9886): 1694–1700. doi:10.1016/S0140-6736(13)60856-9. PMC 4010689. PMID 24517156.
External links
 |
|
| Clinical data | |
|---|---|
| Trade names | Forteo/Forsteo, Teribone[1] |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration |
Subcutaneous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 95% |
| Metabolism | Hepatic (nonspecific proteolysis) |
| Elimination half-life | Subcutaneous: 1 hour |
| Excretion | Renal (metabolites) |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ECHA InfoCard | 100.168.733 |
| Chemical and physical data | |
| Formula | C181H291N55O51S2 |
| Molar mass | 4117.72 g/mol g·mol−1 |
| 3D model (JSmol) | |
FORTEO (teriparatide [rDNA origin] injection) contains recombinant human parathyroid hormone (1- 34), and is also called rhPTH (1-34). It has an identical sequence to the 34 N-terminal amino acids(the biologically active region) of the 84-amino acid human parathyroid hormone.
Teriparatide has a molecular weight of 4117.8 daltons and its amino acid sequence is shown below:
 |
Teriparatide (rDNA origin) is manufactured using a strain of Escherichia coli modified by recombinant DNA technology. FORTEO is supplied as a sterile, colorless, clear, isotonic solution in a glass cartridge which is pre-assembled into a disposable delivery device (pen) for subcutaneous injection. Each prefilled delivery device is filled with 2.7 mL to deliver 2.4 mL. Each mL contains 250 mcg teriparatide (corrected for acetate, chloride, and water content), 0.41 mg glacial acetic acid, 0.1 mg sodium acetate (anhydrous), 45.4 mg mannitol, 3 mg Metacresol, and Water for Injection. In addition, hydrochloric acid solution 10% and/or sodium hydroxide solution 10% may have been added to adjust the product to pH 4.
Each cartridge, pre-assembled into a delivery device, delivers 20 mcg of teriparatide per dose each day for up to 28 days.
REFERENCES
1: Lindsay R, Krege JH, Marin F, Jin L, Stepan JJ. Teriparatide for osteoporosis: importance of the full course. Osteoporos Int. 2016 Feb 22. [Epub ahead of print] Review. PubMed PMID: 26902094.
2: Im GI, Lee SH. Effect of Teriparatide on Healing of Atypical Femoral Fractures: A Systemic Review. J Bone Metab. 2015 Nov;22(4):183-9. doi: 10.11005/jbm.2015.22.4.183. Epub 2015 Nov 30. Review. PubMed PMID: 26713309; PubMed Central PMCID: PMC4691592.
3: Babu S, Sandiford NA, Vrahas M. Use of Teriparatide to improve fracture healing: What is the evidence? World J Orthop. 2015 Jul 18;6(6):457-61. doi: 10.5312/wjo.v6.i6.457. eCollection 2015 Jul 18. Review. PubMed PMID: 26191492; PubMed Central PMCID: PMC4501931.
4: Lecoultre J, Stoll D, Chevalley F, Lamy O. [Improvement of fracture healing with teriparatide: series of 22 cases and review of the literature]. Rev Med Suisse. 2015 Mar 18;11(466):663-7. Review. French. PubMed PMID: 25962228.
5: Sugiyama T, Torio T, Sato T, Matsumoto M, Kim YT, Oda H. Improvement of skeletal fragility by teriparatide in adult osteoporosis patients: a novel mechanostat-based hypothesis for bone quality. Front Endocrinol (Lausanne). 2015 Jan 30;6:6. doi: 10.3389/fendo.2015.00006. eCollection 2015. Review. PubMed PMID: 25688232; PubMed Central PMCID: PMC4311704.
6: Wheeler AL, Tien PC, Grunfeld C, Schafer AL. Teriparatide treatment of osteoporosis in an HIV-infected man: a case report and literature review. AIDS. 2015 Jan 14;29(2):245-6. doi: 10.1097/QAD.0000000000000529. Review. PubMed PMID: 25532609; PubMed Central PMCID: PMC4438749.
7: Campbell EJ, Campbell GM, Hanley DA. The effect of parathyroid hormone and teriparatide on fracture healing. Expert Opin Biol Ther. 2015 Jan;15(1):119-29. doi: 10.1517/14712598.2015.977249. Epub 2014 Nov 3. Review. PubMed PMID: 25363308.
8: Yamamoto M, Sugimoto T. [Glucocorticoid and Bone. Beneficial effect of teriparatide on fracture risk as well as bone mineral density in patients with glucocorticoid-induced osteoporosis]. Clin Calcium. 2014 Sep;24(9):1379-85. doi: CliCa140913791385. Review. Japanese. PubMed PMID: 25177011.
9: Chen JF, Yang KH, Zhang ZL, Chang HC, Chen Y, Sowa H, Gürbüz S. A systematic review on the use of daily subcutaneous administration of teriparatide for treatment of patients with osteoporosis at high risk for fracture in Asia. Osteoporos Int. 2015 Jan;26(1):11-28. doi: 10.1007/s00198-014-2838-7. Epub 2014 Aug 20. Review. PubMed PMID: 25138261.
10: Eriksen EF, Keaveny TM, Gallagher ER, Krege JH. Literature review: The effects of teriparatide therapy at the hip in patients with osteoporosis. Bone. 2014 Oct;67:246-56. doi: 10.1016/j.bone.2014.07.014. Epub 2014 Jul 15. Review. PubMed PMID: 25053463.
11: Meier C, Lamy O, Krieg MA, Mellinghoff HU, Felder M, Ferrari S, Rizzoli R. The role of teriparatide in sequential and combination therapy of osteoporosis. Swiss Med Wkly. 2014 Jun 4;144:w13952. doi: 10.4414/smw.2014.13952. eCollection 2014. Review. PubMed PMID: 24896070.
12: Krege JH, Lane NE, Harris JM, Miller PD. PINP as a biological response marker during teriparatide treatment for osteoporosis. Osteoporos Int. 2014 Sep;25(9):2159-71. doi: 10.1007/s00198-014-2646-0. Epub 2014 Mar 6. Review. PubMed PMID: 24599274; PubMed Central PMCID: PMC4134485.
13: Nakano T. [Once-weekly teriparatide treatment on osteoporosis]. Clin Calcium. 2014 Jan;24(1):100-5. doi: CliCa1401100105. Review. Japanese. PubMed PMID: 24369286.
14: Yano S, Sugimoto T. [Daily subcutaneous injection of teriparatide : the progress and current issues]. Clin Calcium. 2014 Jan;24(1):35-43. doi: CliCa14013543. Review. Japanese. PubMed PMID: 24369278.
15: Lewiecki EM, Miller PD, Harris ST, Bauer DC, Davison KS, Dian L, Hanley DA, McClung MR, Yuen CK, Kendler DL. Understanding and communicating the benefits and risks of denosumab, raloxifene, and teriparatide for the treatment of osteoporosis. J Clin Densitom. 2014 Oct-Dec;17(4):490-5. doi: 10.1016/j.jocd.2013.09.018. Epub 2013 Oct 25. Review. PubMed PMID: 24206867.
16: Delivanis DA, Bhargava A, Luthra P. Subungual exostosis in an osteoporotic patient treated with teriparatide. Endocr Pract. 2013 Sep-Oct;19(5):e115-7. doi: 10.4158/EP13040.CR. Review. PubMed PMID: 23757619.
17: Borges JL, Freitas A, Bilezikian JP. Accelerated fracture healing with teriparatide. Arq Bras Endocrinol Metabol. 2013 Mar;57(2):153-6. Review. PubMed PMID: 23525295.
18: Thumbigere-Math V, Gopalakrishnan R, Michalowicz BS. Teriparatide therapy for bisphosphonate-related osteonecrosis of the jaw: a case report and narrative review. Northwest Dent. 2013 Jan-Feb;92(1):12-8. Review. PubMed PMID: 23516715.
19: Lamy O. [Bone anabolic treatment with Teriparatide]. Ther Umsch. 2012 Mar;69(3):187-91. doi: 10.1024/0040-5930/a000272. Review. German. PubMed PMID: 22403112.
20: Narváez J, Narváez JA, Gómez-Vaquero C, Nolla JM. Lack of response to teriparatide therapy for bisphosphonate-associated osteonecrosis of the jaw. Osteoporos Int. 2013 Feb;24(2):731-3. doi: 10.1007/s00198-012-1918-9. Epub 2012 Mar 8. Review. PubMed PMID: 22398853.
/////TERIPARATIDE, テリパラチド , терипаратид , تيريباراتيد , 特立帕肽 , PTH 1-34, LY 333334, LY-333334, LY333334, ZT-034, 52232-67-4, PEPTIDES



