GSK2334470
GSK2334470; 1227911-45-6; GSK-2334470; GSK 2334470;
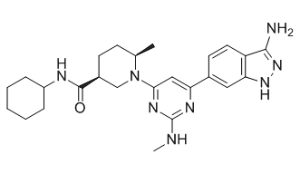
(3S,6R)-1-[6-(3-Amino-1H-indazol-6-yl)-2-(methylamino)-4-pyrimidinyl]-N-cyclohexyl-6-methyl-3-piperidinecarboxamide
(3S.6/?V1-r6-(3-Amino-1 H-indazol-6-ylV2-(methylaminoV4-pyrimidinyll-Λ/-cvclohexyl-6- methyl-3-piperidinecarboxamide
| Molecular Weight | 462.59 |
| Formula | C25H34N8O |
| CAS Number | 1227911-45-6 |

Phosphoinositide Dependent Kinase (PDK) 1 Inhibitors
[α]20D = – 32.6 o (c 1.17, MeOH)
[α] D = -27.6 (Concentration = 1.16, Solvent = Methanol)
SOL………DMSO to 100 mM
ethanol to 100 mM
nmr……http://www.chemietek.com/Files/Line2/Chemietek,%20GSK2334470%20(1),%20NMR-DMSO.pdf
http://file.selleckchem.com/downloads/nmr/S708702-GSK2334470-HNMR-Selleck.pdf

GSK2334470 is a potent and selective PDK1 (3-Phosphoinositide dependent protein kinase-1) inhibitor. GSK2334470 blocks the phosphorylation of known PDK1 substrates, but surprisingly find that the potency and kinetics of inhibition vary for different PDK1 targets. GSK2334470 subsequent activation of PDK1 substrates S6K1, SGK and RSK in HEK293, U87 and mouse embryonic fibroblast cell lines.
GSK2334470 inhibited activation of an Akt1 mutant lacking the PH domain (pleckstrin homology domain) more potently than full-length Akt1, suggesting that GSK2334470 is more effective at inhibiting PDK1 substrates that are activated in the cytosol rather than at the plasma membrane. GSK2334470 also suppressed T-loop phosphorylation and activation of RSK2 (p90 ribosomal S6 kinase 2), another PDK1 target activated by the ERK (extracellular-signal-regulated kinase) pathway.
GSK2334470 is a highly specific and potent inhibitor of PDK1 (3-Phosphoinositide dependent protein kinase-1) with IC50 of 10 nM. It does not suppress activity on other 96 kinases, including Aurora, ROCK, p38 MAPK and PI3K. GSK2334470 has been used in cells to ablate T-loop phosphorylation and activate SGK, S6K1 and RSK as well as suppress the activation of Akt.
PATENT
WO 2010059658
http://www.google.com/patents/WO2010059658A1?cl=en
Example 78
(3S.6/?V1-r6-(3-Amino-1 H-indazol-6-ylV2-(methylaminoV4-pyrimidinyll-Λ/-cvclohexyl-6- methyl-3-piperidinecarboxamide
To (3S,6R)-1-[6-(4-cyano-3-fluorophenyl)-2-(methylamino)-4-pyrimidinyl]-Λ/-cyclohexyl-6- methyl-3-piperidinecarboxamide (260 mg, 0.58 mmol) in EtOH (10 ml.) as a suspension at room temperature in a microwave vial was added hydrazine monohydrate (807 uL, 16.7 mmol, 30 equiv) in one portion. The mixture was capped and heated at 100 0C for 48 hours. A duplicate run was performed. The crude reactions from both runs were combined, and concentrated in vacuo. The residue was taken up in 10 ml. of water. The resulting suspension was sonicated briefly, and filtered. The solids collected were dried under vacuum at room temperature over P2O5 for 18 hours, and then at 65 0C under vacuum for another 18 hours to afford the title compound (410 mg) as a cream-colored solid. LC-MS (ES) m/z = 463 [M+H]+. 1H NMR (400 MHz, CD3OD): δ 1.16 – 1.32 (m, 3H),1.29 (d, J = 6.8 Hz, 3H), 1.34 – 1.45 (m, 2H), 1.65 – 1.68 (m, 1 H), 1.76 – 1.81 (m, 5H), 1.85 – 1.92 (m, 2H), 1.97 – 2.05 (m, 1 H), 2.35 – 2.42 (m, 1 H), 2.97 (s, 3H), 3.1 1 – 3.15 (m, 1 H),3.64 – 3.70 (m, 1 H), 4.45 – 4.65 (bs, 1 H), 4.72 – 4.92 (bs, 1 H), 6.45 (s, 1 H), 7.52 (dd, J =8.5, 1.14 Hz, 1 H), 7.75 (d, J = 8.3 Hz, 1 H), 7.85 (s, 1 H).
ntermediate 112
Cis- methyl-6-methyl-3-piperidinecarboxylate

A solution of cis-3-methyl 1-(phenylmethyl)-6-methyl-1 ,3-piperidinedicarboxylate (69 g, 237 mol) in EtOH (50 mL) and EtOAc (300 mL) was added to a slurry of 10% Pd/C (3.7 g) in EtOAc (30 mL) and EtOH (10 mL) EtOH under nitrogen in a Parr Shaker bottle. The mixture was hydrogenated under 65 psi at room temperature for 4 hours. The mixture was filtered through celite, and washed with EtOAc. The filtrate was concentrated in vacuo to give 37 g of the title compound as a liquid. LC-MS (ES) m/z = 158 [M+H]+.
Intermediate 113
Methyl (3S,6f?)-6-methyl-3-piperidinecarboxylate L-(+)-tartaric acid salt 
L-(+)-Tartaric acid salt A suspension of L-(+)-tartaric acid (39 g, 260 mmol, 1.05 equiv) in IPA (200 ml.) and water (13 mL) water was heated in a water bath at 600C until all dissolved. To this hot stirred solution was added neat racemic methyl (3S,6R)-6-methyl-3-piperidinecarboxylate (39 g, 248 mmol), followed by addition of 25 mL of IPA rinse. The resulting mixture was heated to 60 0C, resulting in a clear solution, and then cooled to room temperature, while the hot water bath was removed. This hot solution was seeded with a sample of methyl (3S,6R)-6-methyl-3-piperidinecarboxylate L-(+)-tartaric acid salt that had a chiral purity of 98% ee, and aged at ambient temperature (with the water bath removed) for 20 minutes. The mixture turned into an oily texture with seeds still present. To the mixture was added 5 mL of water, and heated in the warm water bath at 43 0C. The mixture became clear with the seeds still present. The heating was stopped, and the mixture was stirred in the warm water bath. After 20 minutes, the mixture gradually turned into a paste. After another 10 min, the water bath was removed, and the mixture was stirred at ambient temperature for another 1 hour. The resulting paste was filtered. The cake was washed with 50 mL of IPA, giving 62 g of wet solids. This cake was taken up in 150 mL of IPA and 8 mL of water, and stirred as a slurry while being heated in a water bath to 60 0C (internal temp 55 0C) for 5 minutes. The heating was turned off while the mixture was still stirred in the warm water bath. After 30 min, the mixture was filtered. The cake was washed with 100 mL of IPA. Drying under house vacuum at room temperature for 48 hours gave 46.7 g of solids. An analytical sample was derivatised to the corresponding N-Cbz derivative (as in the preparation of intermediate 1 11 ), which was determined by chiral HPLC (methods used to analyze the resolution of intermediate 11 1 above) to have 85% ee. This material was taken up in IPA (420 mL) and water (38 mL) as a suspension. The mixture was heated in a water bath to 65 0C, at which time the mixture became a clear solution. The heating bath was removed. The mixture was seeded and aged at ambient temp for 20 hours. The solids formed were filtered, and washed with 100 mL of IPA. The solids collected were dried under house vacuum at room temperature for 24 h, and then under vacuum at room temperature for another 24 hours to give 28.5 g of the title compound. An analytical sample was converted to the N-Cbz derivative. The ee was determined to be 97.7%. LC-MS (ES) m/z = 158 [M+H]+.
Intermediate 114 4,6-Dichloro-Λ/-methyl-2-pyrimidinamine

Methylamine (2M solution, 113 ml_, 217 mmol, 2.05 equiv) was charged to a 1 L 3-neck flask fitted with a magnetic stirrer and a thermometer. The mixture was chilled in an ice bath. To this stirred solution was added via addition funnel a solution of 4,6-dichloro-2-(methylsulfonyl)pyrimidine (25 g, 1 10 mmol) in EtOAc (250 ml.) portionwise over a 25 minutes period. The temp was between 5-10 0C. After completion of addition, the ice bath was removed, and the mixture was stirred for 1 hour at ambient temperature. LCMS showed conversion complete. The suspension was filtered, and washed with EtOAc. The filtrate was concentrated in vacuo. The residue was partitioned between water (100 ml.) and EtOAc (450 ml_). The organic was washed with brine, dried over MgSO4, filtered and concentrated in vacuo to give white solids, which were triturated in 150 ml. of CH2CI2. These solids were collected by filtration and washing with cold CH2CI2 (50 ml_). Drying under house vacuum at room temperature for 20 hours, and then high vacuum at room temperature for 3 hours gave 9.31 g of the title compound as a solid. LC-MS (ES) m/z = 179 [M+H]+.
Intermediate 121 (3S,6/?)-1-r6-Chloro-2-(methylamino)-4-pyrimidinyll-Λ/-cvclohexyl-6-methyl-3-piperidinecarboxamide

To a suspension of (3S,6/?)-1-[6-chloro-2-(methylamino)-4-pyrimidinyl]-6-methyl-3-piperidinecarboxylic acid (3.05 g, 10.71 mmol) in CH2CI2 (50 ml.) at room temperature was added Hunig’s base (2.70 ml_, 15.43 mmol, 1.3 equiv) and cyclohexylamine (1.60 ml_, 14.2 mmol, 1.2 equiv), and the resulting mixture was chilled in an ice bath. To this stirred solution was added HATU (4.96 g, 13.1 mmol, 1.1 equiv) in one portion, and the resulting suspension was stirred in the ice bath for 30 minutes. LCMS showed conversion complete. The mixture was diluted with CH2CI2 (50 ml.) and filtered through celite. The filtrate was washed water (2 X 25 ml.) and then brine. The organic was dried over Na2SO4, filtered, and concentrated in vacuo. Silica gel column chromatography using gradient elution of 1 % EtOAc in CHCI3 to 50% EtOAc in CHCI3 afforded the title compound (4.26 g) as a foam. LC-MS (ES) m/z = 366 [M+H]+.
PAPER
Journal of Medicinal Chemistry (2011), 54(6), 1871-1895.
http://pubs.acs.org/doi/full/10.1021/jm101527u
Phosphoinositide-dependent protein kinase-1(PDK1) is a master regulator of the AGC family of kinases and an integral component of the PI3K/AKT/mTOR pathway. As this pathway is among the most commonly deregulated across all cancers, a selective inhibitor of PDK1 might have utility as an anticancer agent. Herein we describe our lead optimization of compound 1toward highly potent and selective PDK1 inhibitors via a structure-based design strategy. The most potent and selective inhibitors demonstrated submicromolar activity as measured by inhibition of phosphorylation of PDK1 substrates as well as antiproliferative activity against a subset of AML cell lines. In addition, reduction of phosphorylation of PDK1 substrates was demonstrated in vivo in mice bearing OCl-AML2 xenografts. These observations demonstrate the utility of these molecules as tools to further delineate the biology of PDK1 and the potential pharmacological uses of a PDK1 inhibitor.
REFERENCES
Najafov, et al., Characterization of GSK2334470, a novel and highly specific inhibitor of PDK1. Biochem.J. (2011), 433 (2) 357.
For a PDK1 inhibitor, the substrate matters.
Knight ZA. Biochem J. 2011 Jan 15;433(2):e1-2. PMID: 21175429.
Characterization of GSK2334470, a novel and highly specific inhibitor of PDK1.
Najafov A, et al. Biochem J. 2011 Jan 15;433(2):357-69. PMID: 21087210.

Jeffrey Axten
Director, Medicinal Chemistry, Virtual Proof of Concept DPU at GlaxoSmithKline
/////
Filed under: Preclinical drugs, Uncategorized Tagged: GSK 2334470, GSK2334470, preclinical


