L-Methyldopa
- Molecular FormulaC10H13NO4
- Average mass211.214 Da
| Name | : | Methyldopa |
| Synonym | : | 3-hydroxy-alpha-methyl-L-tyrosine |
| Mol Formula | : | C10H13NO4 |
| CAS | : | 555-30-6 |
Synthesis ReferenceVincenzo Cannata, Giancarlo Tamerlani, Mauro Morotti, “Process for the synthesis of the levodopa.” U.S. Patent US4962223, issued December, 1986.
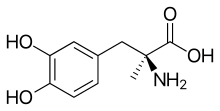
Methyldopa USP is the L-isomer of alpha-methyldopa. Its chemical name is levo-3-(3,4-dihydroxyphenyl)- 2-methylalanine sesquihydrate. Its structural formula is:

C 10H13NO4 • 1 1/2 H2O M.W. 238.24
Levodopa is a prodrug of dopamine that is administered to patients with Parkinson’s due to its ability to cross the blood-brain barrierLabel. Levodopa can be metabolised to dopamine on either side of the blood-brain barrier and so it is generally administered with a dopa decarboxylase inhibitor like carbidopa to prevent metabolism until after it has crossed the blood-brain barrierLabel,1. Once past the blood-brain barrier, levodopa is metabolized to dopamine and supplements the low endogenous levels of dopamine to treat symptoms of Parkinson’sLabel. The first developed drug product that was approved by the FDA was a levodopa and carbidopa combined product called Sinemet that was approved on May 2, 19751,7.
Methyldopa, sold under the brand name Aldomet among others, is a medication used for high blood pressure.[1] It is one of the preferred treatments for high blood pressure in pregnancy.[1] For other types of high blood pressure including very high blood pressure resulting in symptoms other medications are typically preferred.[1] It can be given by mouth or injection into a vein.[1] Onset of effects is around 5 hours and they last about a day.[1]
Common side effects include sleepiness.[1] More severe side effects include red blood cell breakdown, liver problems, and allergic reactions.[1] Methyldopa is in the alpha-2 adrenergic receptor agonist family of medication.[1] It works by stimulating the brain to decrease the activity of the sympathetic nervous system.[1]
Methyldopa was discovered in 1960.[2] It is on the World Health Organization’s List of Essential Medicines, the most effective and safe medicines needed in a health system.[3] The wholesale cost in the developing world is about US$4.31–9.48 per month.[4] In the United States it costs less than $25 per month.[5]
Medical uses
Methyldopa is used in the clinical treatment of the following disorders:
- Hypertension (or high blood pressure)
- Gestational hypertension (or pregnancy-induced hypertension) and pre-eclampsia
Side effects
Methyldopa is capable of inducing a number of adverse side effects, which range from mild to severe. Nevertheless, they are generally mild when the dose is less than 1 gram per day.[6] Side effects may include:
- Psychological
- Depression or even suicidal ideation, as well as nightmares
- Apathy or anhedonia, as well as dysphoria
- Anxiety, especially of the social anxiety variant
- Decreased alertness, awareness, and wakefulness
- Impaired attention, focus, and concentration
- Decreased desire, drive, and motivation
- Fatigue or lethargy or malaise or lassitude
- Sedation or drowsiness or somnolence or sleepiness
- Agitation or restlessness
- Cognitive and memory impairment
- Derealization or depersonalization, as well as mild psychosis
- Sexual dysfunction including impaired libido, desire, and drive
- Physiological
- Dizziness, lightheadedness, or vertigo
- Miosis or pupil constriction
- Xerostomia or dry mouth
- Gastrointestinal disturbances such as diarrhea or constipation
- Headache or migraine
- Myalgia or muscle aches, arthralgia or joint pain, or paresthesia (“pins and needles”)
- Restless legs syndrome (RLS)
- Parkinsonian symptoms such as muscle tremors, rigidity, hypokinesia, or balance or postural instability
- Akathisia, ataxia, dyskinesia as well as even tardive dyskinesia, or dystonia
- Bell’s palsy or facial paralysis
- Sexual dysfunction consisting of impaired erectile dysfunction or anorgasmia
- Hyperprolactinemia or excess prolactin, gynecomastia/breast enlargement in males, or amenorrhoea or absence of menstrual cycles in females
- Bradycardia or decreased heart rate
- Hypotension or decreased blood pressure (though this may also be considered a therapeutic benefit)
- Orthostatic hypotension (also known as postural hypotension)
- Hepatitis, hepatotoxicity, or liver dysfunction or damage
- Pancreatitis or inflammation of the pancreas
- Warm autoimmune hemolytic anemia or deficiency in red blood cells (RBCs)
- Myelotoxicity or bone marrow suppression, potentially leading to thrombocytopenia or blood platelet deficiency or leukopenia or white blood cell (WBC) deficiency
- Hypersensitivity such as lupus erythematosus, myocarditis, or pericarditis
- Lichenoid reactions such as skin lesions or rashes
- Pallor
Rebound/withdrawal
Rebound hypertension via withdrawal on account of tolerance upon the abrupt discontinuation of methyldopa has been reported.[7]
Mechanism of action
Methyldopa has a dual mechanism of action:
- It is a competitive inhibitor of the enzyme DOPA decarboxylase, also known as aromatic L-amino acid decarboxylase, which converts L-DOPA into dopamine. Dopamine is a precursor for norepinephrine (noradrenaline) and subsequently epinephrine (adrenaline). This inhibition results in reduced dopaminergic and adrenergic neurotransmission in the peripheral nervous system. This effect may lower blood pressure and cause central nervous system effects such as depression, anxiety, apathy, anhedonia, and parkinsonism. In addition, decreased dopamine may reduce its inhibitory effect on prolactin leading to signs and symptoms of hyperprolactinemia.
- It is converted to α-methylnorepinephrine by dopamine beta-hydroxylase (DBH). α-Methylnorepinephrine is an agonist of presynaptic central nervous system α2 adrenergic receptors. Activation of these receptors in the brainstem appears to inhibit sympathetic nervous system output and lower blood pressure. This is also the mechanism of action of clonidine.
Pharmacokinetics
Methyldopa exhibits variable absorption from the gastrointestinal tract. It is metabolized in the liver and intestines and is excreted in urine.
History
When methyldopa was first introduced, it was the mainstay of antihypertensive treatment, but its use has declined on account of relatively severe adverse side effects, with increased use of other safer and more tolerable agents such as alpha blockers, beta blockers, and calcium channel blockers. Additionally, it has yet to be associated with reducing adverse cardiovascular events including myocardial infarction and stroke, or overall all-cause mortality reduction in clinical trials.[8] Nonetheless, one of methyldopa’s still current indications is in the management of pregnancy-induced hypertension (PIH), as it is relatively safe in pregnancy compared to many other antihypertensives which may affect the fetus.
PATENT
https://patents.google.com/patent/CN105693541B/en
L-methyldopa an α2 receptor agonistic cardiovascular drugs. The structural formula is as follows:
[0003]
[0004] The product produced methyl α- demethylated metabolite of norepinephrine, blockade of central α receptor, thereby inhibiting the heart, kidney and peripheral vascular sympathetic drive output at the same time, peripheral vascular resistance in vivo and plasma renin activity is reduced, and thus decrease blood pressure. It can be used for treating hypertension, nephropathy, including hypertension time.This product is safe, is the preferred treatment during pregnancy with hypertension drugs.
[0005] In the prior art, methyldopa synthesis are the following:
[0006] 1 to veratridine-one was synthesized by the synthesis of L-hydantoin intermediate methyldopa:
[0007]
[0008] 2 to veratridine-one was synthesized by the synthesis of L-amino nitrile intermediate methyldopa:
[0009]
[0010] 3, eugenol synthesized from L-methyldopa
[0011]
[0012] The plurality of reaction have their own advantages, but in general, the reaction need to use highly toxic cyanide, have a certain impact on the environment and operating conditions.
SUMMARY
[0013] The present invention discloses a method for synthesizing methyldopa, the synthetic route without using highly toxic substances, the advantage of having a clean environment and efficient.
[00 M] methyldopa synthesis method disclosed in the present invention, is 3,4-dimethoxybenzaldehyde with 2-acetylamino-propionic acid methyl ester as a starting material synthesized by condensation, reduction, deprotection to give the crude product methyldopa, methyldopa and then purified to give pure product.Scheme with easy operation, high yield, etc. cleaning process.
[0015] The scheme is as follows:
[0016]
Example 1
[0034] (A) Weigh 3,4_-dimethoxybenzaldehyde 16.6g (0. Imol), sodium methoxide 5.4g (0. Imol), into dried dimethylformamide (150ml), stirring dissolution was complete, the reactor was placed in a cold water bath controlled at a temperature of about 20 ° C, weighed 2-acetamido-methyl 14.5g (0. Imol), successively portionwise added to the reactor, the reaction was stirred for at least 5 minutes plus complete. After all was added, maintaining the reaction temperature for 1.5h.After completion of the reaction, cold water was added Intermediate precipitated solid was filtered and washed several times with cold water.
[0035] (B) Intermediate (A) obtained was transferred to the reactor, 150ml of dichloromethane was added to dissolve, was added p-toluenesulfonyl chloride 19. Ig (0. Imol), triethylamine reactor after 10. Ig (0. Imol), the reaction was stirred for 2h, sodium boron hydride was added 4g, reaction was continued for lh. After completion of the reaction, cold water was added and sufficiently stirred, the aqueous layer was discarded liquid separation, the organic layer, the solvent was evaporated under reduced pressure to obtain an intermediate;
[0036] (C) Intermediate (B) obtained was transferred to the reactor was added 150ml 47% aqueous hydrobromic acid to the reactor, warmed to about 60 ° C, the reaction was stirred at reflux for 4h. Hydrobromic acid was distilled off under reduced pressure to about IlOml, filtered, the mother liquor was concentrated to dryness under reduced pressure, dissolve the solid with cold water, and ammonia to adjust the pH to 4.5 with a cold water bath, the precipitated white solid was large. Filtered and the solid washed with a little cold methylene chloride to give 20.8 g of crude product methyldopa, yield 98.5%.
[0037] (D) The crude product take methyldopa, add 30ml 0. Imo 1 / L dilute hydrochloric acid, an Ig activated carbon, heated, stirred until dissolution methyldopa, maintaining the temperature 〇.5h, filtered hot and allowed to cool to ammonia to adjust the pH to 4.5 to precipitate large amount of white solid was filtered, rinsed with a small amount of cold water, and dried to give 17.7 g methyldopa pure, a yield of 85.0%. Content was determined according to the “Chinese Pharmacopoeia” method and its content was 99.6%.
[0038] Example 2
[0039] (A) Weigh 166kg of 3,4-dimethoxybenzaldehyde, 54kg sodium methoxide, into dried dimethylformamide 500L stirred to dissolve completely, the reactor was placed in a cold water bath controlled temperature of about 20 ° C, 2-acetamido-Weigh 145kg methyl, successively portionwise added to the reactor, the reaction was stirred for at least 5 minutes the addition was completed. After all was added, maintaining the reaction temperature for 2 h. After completion of the reaction, cold water was added Intermediate precipitated solid was filtered and washed several times with cold water.
[0040] (B) Intermediate (A) obtained was transferred to the reactor, 500L of methylene chloride was added to dissolve, was added p-toluenesulfonyl chloride 19Ikg, triethylamine 10Ikg into the reactor, the reaction was stirred for 2.5h , sodium borohydride was added 4kg, reaction was continued for 1.3h. After completion of the reaction, cold water was added and sufficiently stirred, the aqueous layer was discarded liquid separation, the organic layer, the solvent was evaporated under reduced pressure to obtain an intermediate;
[0041] (C) Intermediate (B) obtained was transferred to the reactor, was added 500L 47% aqueous hydrobromic acid to the reactor, warmed to about 60 ° C, the reaction was stirred at reflux for 4h. Hydrobromic acid was distilled off under reduced pressure to about 375L, filtered, and the mother liquor was concentrated to dryness under reduced pressure, dissolve the solid with cold water, and ammonia to adjust the pH to 4.5 with a cold water bath, the precipitated white solid was large. Centrifuged, and the solid washed with a little cold water to give the crude product methyldopa 200kg, 94.7% yield.
[0042] (D) The crude product take methyldopa, add 300L O.lmol / L dilute hydrochloric acid, 10 kg activated carbon, heating and stirring until dissolved methyldopa, maintaining the temperature 〇.5h, filtered hot and allowed to cool to ammonia to adjust the pH to 4.5 to precipitate large amount of white solid was filtered, rinsed with a small amount of cold water, and dried to give pure methyldopa 177kg, 88.5% yield. Content was determined according to the “Chinese Pharmacopoeia” method and its content was 99.7%.
CLIP

Exists as the sesquihydrate.
Prepn: Pfister, Stein, US 2868818 (1959 to Merck & Co.). D. F. Reinhold and M. Sletzinger, GB 936074 eidem U.S. Patent 3,344,023 (1963 to Merck and Co.)
Resolution: Jones et al., US 3158648 (1964 to Merck & Co.); cf. Slates et al., J. Org. Chem. 29, 1424 (1964). Resolution and configuration: Tristram, ibid. 2053.
Synthesis from asymmetric intermediates: Reinhold et al., J. Org. Chem. 33, 1209 (1968).
Prepn of the ethyl ester hydrochloride: FR M2153 (1963 to Merck & Co.);
of pharmaceutical dosage forms: Marcus, US 3230143 (1966 to Merck & Co.).
CLIP
Reactions of D-glucose with phenolic amino acids: further insights into the competition between Maillard and Pictet-Spengler condensation pathways
Carbohydrate Research (2005), 340, (18), 2719-2727
Methyldopa
-
- ATC:C02AB01
- Use:antihypertensive
- Chemical name:3-hydroxy-α-methyl-l-tyrosine
- Formula:C10H13NO4
- MW:211.22 g/mol
- CAS-RN:555-30-6
- InChI Key:CJCSPKMFHVPWAR-JTQLQIEISA-N
- InChI:InChI=1S/C10H13NO4/c1-10(11,9(14)15)5-6-2-3-7(12)8(13)4-6/h2-4,12-13H,5,11H2,1H3,(H,14,15)/t10-/m0/s1
- EINECS:209-089-2
Synthesis
References
-
- Tristram, E.W. et al.: J. Org. Chem. (JOCEAH) 29, 2053 (1964).
- B Stein, G.A. et al.: J. Am. Chem. Soc. (JACSAT) 77, 700 (1955).
- Chem. Eng. from 8.11.1965; p. 247.
- C Reinhold, D.F. et al.: J. Org. Chem. (JOCEAH) 33, 1209 (1968).
- A US 2 868 818 (Merck & Co.; 13.1.1959; prior. 15.12.1953).
- GB 936 074 (Merck & Co.; appl. 18.10.1960; USA-prior. 8.4.1960, 24.8.1960).
- DE 1 171 931 (Merck & Co.; prior. 6.10.1960).
- US 3 158 648 (Merck & Co.; 24.11.1964; prior. 11.7.1961, 9.4.1962).
- FR 1 492 765 (Merck & Co.; appl. 10.10.1963; USA-prior. 11.10.1962, 19.9.1963).
-
similar method via l-α-acetylamino-α-vanillylpropionitrile:
- GB 1 142 595 (Merck & Co.; appl. 23.5.1967, 12.2.1969).
-
alternative syntheses:
- D a Steinetal, G.A.: J. Am. Chem. Soc. (JACSAT) 77, 700 (1955).
- US 3 366 679 (Merck & Co.; 30.1.1968; prior. 11.10.1962, 19.9.1963).
- DOS 2 302 937 (Tanabe; appl. 22.1.1973; J-prior. 22.1.1972).
- US 3 517 057 (Merck & Co.; 23.6.1970; appl. 21.9.1967).
- DE 1 235 946 (Boehringer Mannh.; appl. 8.8.1964).
- DE 1 235 947 (Bayer; appl. 16.1.1963).
- DE 1 258 416 (Knoll; appl. 9.10.1964).
- DE 1 269 622 (Knoll; appl. 22.12.1966).
- DOS 2 406 898 (BASF; appl. 14.2.1974).
- AT 250 936 (Egyesült; appl. 3.11.1964; HU-prior. 18.11.1963).
- FR 1 502 972 (Merck & Co.; appl. 21.10.1966; USA-prior. 22.10.1965).
- FR 1 531 877 (Sankyo; appl. 18.7.1967; J-prior. 11.8.1966, 21.2.1967).
- GB 1 321 802 (D.D.S.A.; appl. 5.2.1971).
- b GB 2 059 955 (Merck & Co.; appl. 9.9.1980; USA-prior. 13.9.1979, 28.9.1979).
-
medical use:
- US 3 344 023 (Merck & Co.; 12.4.1983; prior. 8.4.1960, 24.8.1960, 1.2.1963; reexamination request 21.12.1981).
-
References
- ^ Jump up to:a b c d e f g h i j k “Methyldopa”. The American Society of Health-System Pharmacists. Archived from the original on 21 December 2016. Retrieved 8 December 2016.
- ^ Walker, S. R. (2012). Trends and Changes in Drug Research and Development. Springer Science & Business Media. p. 109. ISBN 9789400926592. Archived from the original on 2016-09-14.
- ^ “WHO Model List of Essential Medicines (19th List)” (PDF). World Health Organization. April 2015. Archived (PDF) from the original on 13 December 2016. Retrieved 8 December 2016.
- ^ “Methyldopa”. International Drug Price Indicator Guide. Retrieved 8 December 2016.
- ^ Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 140. ISBN 9781284057560.
- ^ British National Formulary 56. September 2008. pp. 95–96. ISBN 978-0-85369-778-7.
- ^ Methyldopa (PIM 342) Archived 2008-03-13 at the Wayback Machine
- ^ Mah GT, Tejani AM, Musini VM. Methyldopa for primary hypertension. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No.: CD003893. DOI: 10.1002/14651858.CD003893.pub3.
External links
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Aldomet, Aldoril, Dopamet, others |
| Synonyms | L-α-Methyl-3,4-dihydroxyphenylalanine |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682242 |
| Pregnancy category |
|
| Routes of administration |
by mouth, IV |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | approximately 50% |
| Metabolism | Liver |
| Onset of action | 4 to 6 hrs[1] |
| Elimination half-life | 105 minutes |
| Duration of action | 10 to 48 hrs[1] |
| Excretion | Kidney for metabolites |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.008.264 |
| Chemical and physical data | |
| Formula | C10H13NO4 |
| Molar mass | 211.215 g/mol g·mol−1 |
| 3D model (JSmol) | |
Other Names
- Alanine, 3-(3,4-dihydroxyphenyl)-2-methyl-, L- (8CI)
- 3-Hydroxy-α-methyl-L-tyrosine
- (-)-Methyldopa
- (-)-α-Methyl-3,4-dihydroxyphenylalanine
- (-)-α-Methyldopa
- (2S)-2-Amino-3-(3,4-dihydroxyphenyl)-2-methylpropanoic acid
- (S)-(-)-α-Methyldopa
- (S)-α-Methyldopa
- 2-Methyl-3-(3,4-dihydroxyphenyl)alanine
- AMD
- Aldochlor
- Aldomet
- Aldometil
- Aldomin
- Aldomine
- Alpha medopa
- Alphamethyldopa
- Bayer 1440L
- Baypresol
- Dopamet
- Dopatec
- Dopegyt
- Elanpres
- Equibar
- L-(-)-α-Methyl-β-(3,4-dihydroxyphenyl)alanine
- L-(-)-β-(3,4-Dihydroxyphenyl)-α-methylalanine
- L-2-Amino-2-methyl-3-(3,4-dihydroxyphenyl)propionic acid
- L-3,4-Dihydroxy-α-methylphenylalanine
- L-3,4-Dihydroxyphenyl-2-methylalanine
- L-Methyldopa
- L-α-Methyl-3,4-dihydroxyphenylalanine
- L-α-Methyl-3-(3,4)-dihydroxyphenylalanine
- L-α-Methyldopa
- Lederdopa
- Levo-3-(3,4-Dihydroxyphenyl)-2-methylalanine
- MK 351
- Medomet
- Medopa
- Medopren
- Methoplain
- Methyl-L-dopa
- Methyldopa
- NSC 169916
- Nr.C 2294
- Presinol
- Presolisin
- Sembrina
- l-3-(3,4-Dihydroxyphenyl)-2-methylalanine
- l-α-Methyldopa
- α-Methyl-L-3,4-dihydroxyphenylalanine
- α-Methyl-L-dopa
- α-Methyldopa
General References
- Djamshidian A, Poewe W: Apomorphine and levodopa in Parkinson’s disease: Two revolutionary drugs from the 1950’s. Parkinsonism Relat Disord. 2016 Dec;33 Suppl 1:S9-S12. doi: 10.1016/j.parkreldis.2016.12.004. Epub 2016 Dec 22. [PubMed:28012951]
- Meiser J, Weindl D, Hiller K: Complexity of dopamine metabolism. Cell Commun Signal. 2013 May 17;11(1):34. doi: 10.1186/1478-811X-11-34. [PubMed:23683503]
- Elroby SA, Makki MS, Sobahi TR, Hilal RH: Toward the understanding of the metabolism of levodopa I. DFT investigation of the equilibrium geometries, acid-base properties and levodopa-water complexes. Int J Mol Sci. 2012;13(4):4321-39. doi: 10.3390/ijms13044321. Epub 2012 Apr 2. [PubMed:22605980]
- Robertson DR, Wood ND, Everest H, Monks K, Waller DG, Renwick AG, George CF: The effect of age on the pharmacokinetics of levodopa administered alone and in the presence of carbidopa. Br J Clin Pharmacol. 1989 Jul;28(1):61-9. [PubMed:2775615]
- Abrams WB, Coutinho CB, Leon AS, Spiegel HE: Absorption and metabolism of levodopa. JAMA. 1971 Dec 27;218(13):1912-4. [PubMed:5171067]
- Fanali G, Rampoldi V, di Masi A, Bolli A, Lopiano L, Ascenzi P, Fasano M: Binding of anti-Parkinson’s disease drugs to human serum albumin is allosterically modulated. IUBMB Life. 2010 May;62(5):371-6. doi: 10.1002/iub.317. [PubMed:20225277]
- FDA Approved Drug Products: Sinemet [Link]
- Sinemet FDA Label [File]
/////////Methyl-L-dopa, Methyldopa, NSC 169916






