LK-01
Leukos Biotech S.L.
APL-130277, H-001, Apokyn

Apomorphine hydrochloride hemihydrate
CAS 41372-20-7
Leukos Biotech (following its spin-off from Jose Carreras Leukaemia Research Institute) is developing LK-01 , a solid form of apomorphine for the sc treatment of acute myeloid leukemia (AML) and the phase II trial results were expected later in 2019.
Apomorphine (brand names Apokyn, Ixense, Spontane, Uprima) is a type of aporphine having activity as a non-selective dopamine agonist which activates both D2-like and, to a much lesser extent, D1-like receptors.[1] It also acts as an antagonist of 5-HT2 and α-adrenergic receptors with high affinity. The compound is historically a morphine decomposition product made by boiling morphine with concentrated acid, hence the –morphine suffix. Contrary to its name, apomorphine does not actually contain morphine or its skeleton, nor does it bind to opioid receptors. The apo– prefix relates to it being a morphine derivative (“[comes] from morphine”).
Historically, apomorphine has been tried for a variety of uses, including as a way to relieve anxiety and craving in alcoholics, an emetic (to induce vomiting), for treating stereotypies (repeated behaviour) in farmyard animals, and more recently in treating erectile dysfunction. Currently, apomorphine is used in the treatment of Parkinson’s disease. It is a potent emetic and should not be administered without an antiemetic such as domperidone. The emetic properties of apomorphine are exploited in veterinary medicine to induce therapeutic emesis in canines that have recently ingested toxic or foreign substances.
Apomorphine was also used as a private treatment of heroin addiction, a purpose for which it was championed by the author William S. Burroughs. Burroughs and others claimed that it was a “metabolic regulator” with a restorative dimension to a damaged or dysfunctional dopaminergic system. There is more than enough anecdotal evidence to suggest that this offers a plausible route to an abstinence-based model; however, no clinical trials have ever tested this hypothesis. A recent study indicates that apomorphine might be a suitable marker for assessing central dopamine system alterations associated with chronic heroin consumption.[2] There is, however, no clinical evidence that apomorphine is an effective and safe treatment regimen for opiate addiction.[3]
Uses
Apomorphine is used in advanced Parkinson’s disease intermittent hypomobility (“off” episodes), where a decreased response to an anti-Parkinson drug such as L-DOPA causes muscle stiffness and loss of muscle control.[4][5] While apomorphine can be used in combination with L-DOPA, the intention is usually to reduce the L-DOPA dosing, as by this stage the patient often has many of dyskinesias caused by L-DOPA and hypermobility periods.[6][7] When an episode sets in, the apomorphine is injected subcutaneously, and signs subside. It is used an average of three times a day.[6] Some people use portable mini-pumps that continuously infuse them with apomorphine, allowing them to stay in the “on” state and using apomorphine as an effective monotherapy.[7][8]
Contraindications
The main and absolute contraindication to using apomorphine is the concurrent use of adrenergic receptor antagonists; combined, they cause a severe drop in blood pressure and fainting.[6][5] Alcohol causes an increased frequency of orthostatic hypotension (a sudden drop in blood pressure when getting up), and can also increase the chances of pneumonia and heart attacks.[6] Dopamine antagonists, by their nature of competing for sites at dopamine receptors, reduce the effectiveness of the agonistic apomorphine.[6][5]
IV administration of apomorphine is highly discouraged, as it can crystallize in the veins and create a blood clot (thrombus) and block a pulmonary artery (pulmonary embolism).[6][5]
Side effects
Nausea and vomiting are common side effects when first beginning therapy with apomorphine;[9] antiemetics such as trimethobenzamide or domperidone, dopamine antagonists,[10] are often used while first starting apomorphine. Around 50% of people grow tolerant enough to apomorphine’s emetic effects that they can discontinue the antiemetic.[5][6]
Other side effects include orthostatic hypotension and resultant fainting, sleepiness, dizziness, runny nose, sweating, paleness, and flushing. More serious side effects include dyskenesias (especially when taking L-DOPA), fluid accumulation in the limbs (edema), suddenly falling asleep, confusion and hallucinations, increased heart rate and heart palpitations, and persistent erections(priaprism).[5][6][11] The priaprism is caused by apomorphine increasing arterial blood supply to the penis. This side effect has been exploited in studies attempting to treat erectile dysfunction.[12]
Pharmacology
Mechanism of action
Apomorphine’s R-enantiomer is an agonist of both D1 and D2 dopamine receptors, with higher activity at D2.[6][10] The members of the D2 subfamily, consisting of D2, D3, and D4receptors, are inhibitory G protein–coupled receptors. The D4 receptor in particular is an important target in the signaling pathway, and is connected to several neurological disorders.[13] Shortage or excess of dopamine can prevent proper function and signaling of these receptors leading to disease states.[14]
Apomorphine improves motor function by activating dopamine receptors in the nigrostriatal pathway, the limbic system, the hypothalamus, and the pituitary gland.[15] It also increases blood flow to the supplementary motor area and to the dorsolateral prefrontal cortex (stimulation of which has been found to reduce the tardive dyskinesia effects of L-DOPA).[16][17]Parkinson’s has also been found to have excess iron at the sites of neurodegeneration; both the R- and S-enantiomers of apomorphine are potent iron chelators and radical scavengers.[10][18]
Apomorphine also reduces the breakdown of dopamine in the brain (though it inhibits its synthesis as well).[19][20] It is a powerful upregulator of certain neural growth factors,[21] in particular NGF and BDNF, epigenetic downregulation of which has been associated with addictive behaviour in rats.[22][23]
Apomorphine causes vomiting by acting on dopamine receptors in the chemoreceptor trigger zone of the medulla; this activates the nearby vomiting center.[15][20][24]
Pharmacokinetics
While apomorphine has lower bioavailability when taken orally, due to not being absorbed well in the GI tract and undergoing heavy first-pass metabolism,[18][8] it has a bioavailability of 100% when given subcutaneously.[6][15] It reaches peak plasma concentration in 10–60 minutes. Ten to twenty minutes after that, it reaches its peak concentration in the cerebrospinal fluid. Its lipophilic structure allows it to cross the blood–brain barrier.[6][15]
Apomorphine possesses affinity for the following receptors (note that a higher Ki indicates a lower affinity):[25][26][27]
| Receptor | Ki (nM) | Action |
|---|---|---|
| D1 | 484 | (partial) agonista |
| D2 | 52 | partial agonist (IA = 79% at D2S; 53% at D2L) |
| D3 | 26 | partial agonist (IA = 82%) |
| D4 | 4.37 | partial agonist (IA = 45%) |
| D5 | 188.9 | (partial) agonista |
| aThough its efficacies at D1 and D5 are unclear, it is known to act as an agonist at these sites.[28] | ||
| Receptor | Ki (nM) | Action |
|---|---|---|
| 5-HT1A | 2,523 | partial agonist |
| 5-HT1B | 2,951 | no action |
| 5-HT1D | 1,230 | no action |
| 5-HT2A | 120 | antagonist |
| 5-HT2B | 132 | antagonist |
| 5-HT2C | 102 | antagonist |
| Receptor | Ki (nM) | Action |
|---|---|---|
| α1A-adrenergic | 1,995 | antagonist |
| α1B-adrenergic | 676 | antagonist |
| α1D-adrenergic | 64.6 | antagonist |
| α2A-adrenergic | 141 | antagonist |
| α2B-adrenergic | 66.1 | antagonist |
| α2C-adrenergic | 36.3 | antagonist |
It has a Ki of over 10,000 nM (and thus negligible affinity) for β-adrenergic, H1, and mACh.[1]
Apomorphine has a high clearance rate (3–5 L/kg/hr) and is mainly metabolized and excreted by the liver.[15] It is likely that while the cytochrome P450 system plays a minor role, most of apomorphine’s metabolism happens via auto-oxidation, O-glucuronidation, O-methylation, N-demethylation, and sulfation.[6][15][20] Only 3–4% of the apomorphine is excreted unchanged and into the urine. The half-life is 30–60 minutes, and the effects of the injection last for up to 90 minutes.[6][7][15]
Toxicity depends on the route of administraion; the LD50s in mice were 300 mg/kg for the oral route, 160 mg/kg for intraperitoneal, and 56 mg/kg intravenous.[29]
Chemistry
Properties
Apomorphine has a catechol structure similar to that of dopamine.[19]
Synthesis
Several techniques exist for the creation of apomorphine from morphine. In the past, morphine had been combined with hydrochloric acid at high temperatures (around 150 °C) to achieve a low yield of apomorphine, ranging anywhere from 0.6% to 46%.[30]
More recent techniques create the apomorphine in a similar fashion, by heating it in the presence of any acid that will promote the essential dehydration rearrangement of morphine-type alkaloids, such as phosphoric acid. The method then deviates by including a water scavenger, which is essential to remove the water produced by the reaction that can react with the product and lead to decreased yield. The scavenger can be any reagent that will irreversibly react with water such as phthalic anhydride or titanium chloride. The temperature required for the reaction varies based upon choice of acid and water scavenger. The yield of this reaction is much higher: at least 55%.[30]
Conversion of Morphine (I) to Apomorphine (II) in the presence of acid following the example of the morphine skeleton dehydration rearrangement, outlined by Bentley.[31]
History
The pharmacological effects of the naturally-occurring analog aporphine in the blue lotus (N. caerulea)[32] were known to the ancient Egyptians and Mayans,[33] with the plant featuring in tomb frescoes and associated with entheogenic rites. It is also observed in Egyptian erotic cartoons, suggesting that they were aware of its erectogenic properties.
The modern medical history of apomorphine begins with its synthesis by Arppe in 1845[34] from morphine and sulfuric acid, although it was named sulphomorphide at first. Matthiesen and Wright (1869) used hydrochloric acid instead of sulfuric acid in the process, naming the resulting compound apomorphine. Initial interest in the compound was as an emetic, tested and confirmed safe by London doctor Samuel Gee,[35] and for the treatment of stereotypies in farmyard animals.[36] Key to the use of apomorphine as a behavioural modifier was the research of Erich Harnack, whose experiments in rabbits (which do not vomit) demonstrated that apomorphine had powerful effects on the activity of rabbits, inducing licking, gnawing and in very high doses convulsions and death.
Treatment of alcoholism
Apomorphine was one of the earliest used pharmacotherapies for alcoholism. The Keeley Cure (1870s to 1900) contained apomorphine, among other ingredients, but the first medical reports of its use for more than pure emesis come from James Tompkins[37] and Charles Douglas.[38][39] Tompkins reported, after injection of 6.5 mg (“one tenth of a grain”):
In four minutes free emesis followed, rigidity gave way to relaxation, excitement to somnolence, and without further medication the patient, who before had been wild and delirious, went off into a quiet sleep.
Douglas saw two purposes for apomorphine:
[it can be used to treat] a paroxysm of dipsomania [an episode of intense alcoholic craving]… in minute doses it is much more rapidly efficient in stilling the dipsomaniac craving than strychnine or atropine… Four or even 3m [minim – roughly 60 microlitres] of the solution usually checks for some hours the incessant demands of the patient… when he awakes from the apomorphine sleep he may still be demanding alcohol, though he is never then so insistent as before. Accordingly it may be necessary to repeat the dose, and even to continue to give it twice or three times a day. Such repeated doses, however, do not require to be so large: 4 or even 3m is usually sufficient.
This use of small, continuous doses (1/30th of a grain, or 2.16 mg by Douglas) of apomorphine to reduce alcoholic craving comes some time before Pavlov‘s discovery and publication of the idea of the “conditioned reflex” in 1903. This method was not limited to Douglas; the Irish doctor Francis Hare, who worked in a sanatorium outside London from 1905 onwards, also used low-dose apomorphine as a treatment, describing it as “the most useful single drug in the therapeutics of inebriety”.[40] He wrote:
In (the) sanatorium it is used in three different sets of circumstances: (1) in maniacal or hysterical drunkenness: (2) during the paroxysm of dipsomania, in order to still the craving for alcohol; and (3) in essential insomnia of a special variety… [after giving apomorphine] the patient’s mental condition is entirely altered. He may be sober: he is free from the time being from any craving from alcohol. The craving may return, however, and then it is necessary to repeat the injection, it may be several times at intervals of a few hours. These succeeding injections should be quite small, 3 to 6 min. being sufficient. Doses of this size are rarely emetic. There is little facial pallor, a sensation as of the commencement of sea-sickness, perhaps a slight malaise with a sudden subsidence of the craving for alcohol, followed by a light and short doze.
He also noted there appeared to be a significant prejudice against the use of apomorphine, both from the associations of its name and doctors being reluctant to give hypodermic injections to alcoholics. In the US, the Harrison Narcotics Tax Act made working with any morphine derivatives extremely hard, despite apomorphine itself not being an opiate.
In the 1950s the neurotransmitter dopamine was discovered in the brain by Kathleen Montagu, and characterised as a neurotransmitter a year later by Arvid Carlsson, for which he would be awarded the Nobel Prize.[41] A. N. Ernst then discovered in 1965 that apomorphine was a powerful stimulant of dopamine receptors.[42] This, along with the use of sublingual apomorphine tablets, led to a renewed interest in the use of apomorphine as a treatment for alcoholism. A series of studies of non-emetic apomorphine in the treatment of alcoholism were published, with mostly positive results.[43][44][45][46][47] However, there was little clinical consequence.
Parkinson’s disease
The use of apomorphine to treat “the shakes” was first suggested by Weil in France in 1884,[48] although seemingly not pursued until 1951.[49] Its clinical use was first reported in 1970 by Cotzias et al.,[50] although its emetic properties and short half-life made oral use impractical. A later study found that combining the drug with the antiemetic domperidoneimproved results significantly.[51] The commercialization of apomorphine for Parkinson’s disease followed its successful use in patients with refractory motor fluctuations using intermittent rescue injections and continuous infusions.[52]
Aversion therapy
Aversion therapy in alcoholism had its roots in Russia in the early 1930s,[53] with early papers by Pavlov, Galant and Sluchevsky and Friken,[54] and would remain a strain in the Soviet treatment of alcoholism well into the 1980s. In the US a particularly notable devotee was Dr Voegtlin,[55] who attempted aversion therapy using apomorphine in the mid to late 1930s. However, he found apomorphine less able to induce negative feelings in his subjects than the stronger and more unpleasant emetic emetine.
In the UK, however, the publication of J Y Dent’s (who later went on to treat Burroughs) 1934 paper “Apomorphine in the treatment of Anxiety States”[56] laid out the main method by which apomorphine would be used to treat alcoholism in Britain. His method in that paper is clearly influenced by the then-novel idea of aversion:
He is given his favourite drink, and his favourite brand of that drink… He takes it stronger than is usual to him… The small dose of apomorphine, one-twentieth of a grain [3.24mg], is now given subcutaneously into his thigh, and he is told that he will be sick in a quarter of an hour. A glass of whisky and water and a bottle of whisky are left by his bedside. At six o’clock (four hours later) he is again visited and the same treatment is again administered… The nurse is told in confidence that if he does not drink, one-fortieth [1.62mg] of a grain of apomorphine should be injected during the night at nine o’clock, one o’clock, and five o’clock, but that if he drinks the injection should be given soon after the drink and may be increased to two hourly intervals. In the morning at about ten he is again given one or two glasses of whisky and water… and again one-twentieth of a grain [3.24mg] of apomorphine is injected… The next day he is allowed to eat what he likes, he may drink as much tea as he likes… He will be strong enough to get up and two days later he leaves the home.
However, even in 1934 he was suspicious of the idea that the treatment was pure conditioned reflex – “though vomiting is one of the ways that apomorphine relives the patient, I do not believe it to be its main therapeutic effect.” – and by 1948 he wrote:[3]
It is now twenty-five years since I began treating cases of anxiety and alcoholism with apomorphine, and I read my first paper before this Society fourteen years ago. Up till then I had thought, and, unfortunately, I said in my paper, that the virtue of the treatment lay in the conditioned reflex of aversion produced in the patient. This statement is not even a half truth… I have been forced to the conclusion that apomorphine has some further action than the production of a vomit.
This led to his development of lower-dose and non-aversive methods, which would inspire a positive trial of his method in Switzerland by Dr Harry Feldmann[57] and later scientific testing in the 1970s, some time after his death. However, the use of apomorphine in aversion therapy had escaped alcoholism, with its use to treat homosexuality leading to the death of a British Army Captain Billy Clegg HIll in 1962,[58] helping to cement its reputation as a dangerous drug used primarily in archaic behavioural therapies.
Opioid addiction
In his Deposition: Testimony Concerning a Sickness in the introduction to later editions of Naked Lunch (first published in 1959), William S. Burroughs wrote that apomorphine treatment was the only effective cure to opioid addiction he has encountered:
The apomorphine cure is qualitatively different from other methods of cure. I have tried them all. Short reduction, slow reduction, cortisone, antihistamines, tranquilizers, sleeping cures, tolserol, reserpine. None of these cures lasted beyond the first opportunity to relapse. I can say that I was never metabolically cured until I took the apomorphine cure… The doctor, John Yerbury Dent, explained to me that apomorphine acts on the back brain to regulate the metabolism and normalize the blood stream in such a way that the enzyme stream of addiction is destroyed over a period of four to five days. Once the back brain is regulated apomorphine can be discontinued and only used in case of relapse.
He goes on to lament the fact that as of his writing, little to no research has been done on apomorphine or variations of the drug to study its effects on curing addiction, and perhaps the possibility of retaining the positive effects while removing the side effect of vomiting.
Despite his claims throughout his life, Burroughs never really cured his addiction and was back to using opiates within years of his apomorphine “cure”.[59] However, he insisted on apomorphine’s effectiveness in several works and interviews.[citation needed]
Society and culture
- Apomorphine has a vital part in Agatha Christie‘s detective story Sad Cypress.
- The 1965 Tuli Kupferberg song “Hallucination Horrors” recommends apomorphine at the end of each verse as a cure for hallucinations brought on by a humorous variety of intoxicants; the song was recorded by The Fugs and appears on the album Virgin Fugs.
Research
There is renewed interest in the use of apomorphine to treat addiction, in both smoking cessation[60] and alcoholism.[61] As the drug is old, out of patent, and safe for use in humans, it is a viable target for repurposing.
Apomorphine has been researched as a possible treatment for erectile dysfunction and female hypoactive sexual desire disorder, though the arousal effects were found not to be reliable enough. One large study found that only 39.4% got erections (compared to baseline 13.1); another found that apomorphine was successful 45–51% of the time, but the placebo also worked 36% of the time.[12][62] Nonetheless, it was under development as a treatment for erectile dysfunction by TAP Pharmaceuticals under the brand name Uprima. In 2000, TAP withdrew its new drug application after an FDA review panel raised questions about the drug’s safety, due to many clinical trial subjects fainting after taking the drug.[63]
Alzheimer’s disease
Apomorphine is reported to be an inhibitor of amyloid beta protein (Aβ) fiber formation, whose presence is a hallmark of Alzheimer’s disease (AD), and a potential therapeutic under the amyloid hypothesis.[64] While it promotes oligomerization of the Aβ40 group of molecules, it inhibits more advanced fibril formation; this is thought to be due to the autoxidation that occurs at the hydroxyl groups. Once this functional group was altered, the inhibitory effect could be seen to decrease, reducing either the indirect or direct interference of the fibril formation.[64]
The protective effects of apomorphine were tested in mouse models with mutations in genes related to AD, such as the amyloid precursor protein gene. Apomorphine was seen to significantly improve memory function through the increased successful completion of the Morris Water Maze. The levels of the aberrant proteins that lead to neuronal disruption were also tested in the brains of mice. Treatment was seen to decrease the intraneuronal levels of the more aggressive Aβ42 molecule when compared to the control mice. This result is consistent with the finding that another protein linked to AD, tau protein, was seen to decrease with apomorphine treatment.[65]
Veterinary use
Apomorphine is used to inducing vomiting in dogs the after ingestion of various toxins or foreign bodies. It can be given subcutaneously, intramuscularly, intravenously, or, when a tablet is crushed, in the conjunctiva of the eye.[66][67] The oral route is ineffective, as apomorphine cannot cross the blood–brain barrier fast enough, and blood levels don’t reach a high enough concentration to stimulate the chemoreceptor trigger zone.[66] It can remove around 40–60% of the contents in the stomach.[68]
One of the reasons apomorphine is a preferred drug is its reversibility:[69] in cases of prolonged vomiting, the apomorphine can be reversed with dopamine antagonists like the phenothiazines (for example, acepromazine). Giving apomorphine after giving acepromazine, however, will no longer stimulate vomiting, because apomorphine’s target receptors are already occupied.[66] An animal who undergoes severe respiratory depression due to apomorphine can be treated with naloxone.[66][67]
Apomorphine does not work in cats, who have too few dopamine receptors.[66]
PATENT
WO-2019141673
Novel crystalline forms of apomorphine or its palmitate or hydrochloride salt useful treating acute myeloid leukemia, Parkinson’s disease, sexual dysfunction and solid tumors. Also claims the process for preparing apomorphine palmitic acid cocrystal salt.
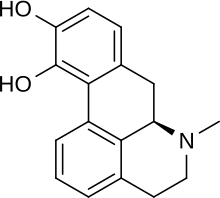
Apomorphine (APO) is a commercial available medical drug with the chemical formula C-17H-17NO2 and structure:
Apomorphine (APO) has been described for treatment of different medical indications – for instance:
– WO2015/197839A1 : leukemia such as acute myeloid leukemia (AML);
– WO2016/103262A2: Parkinson’s disease;
– WO02/39879A2: sexual dysfunction in a patient taking antidepressant medication;
– W02004/082630A2: neurological function of an individual who has a brain injury.
Apomorphine hydrochloride (HCI) is a salt present in commercially available medical products (e.g. APO-Go® PFS or Apokyn®).
A common side effect of administering apomorphine hydrochloride by e.g. subcutaneous injection is e.g. the development of subcutaneous nodules at the injection site, which can become infected, necessitating treatment or surgical involvement.
In relation to this problem – above discussed WO2016/103262A2 describes an alternative solid form of apomorphine, which is e.g. an alcohol solvate crystal of apomorphine free base, wherein the solvate forming solvent is (C-|-C8) alkanol, preferably isopropanol (IPA – i.e. a solid crystalline form of apomorphine-IPA.
Palmitic acid (hexadecanoic acid in IUPAC nomenclature) is a fatty acid found with the chemical formula CH3(CH2)14COOH.
Palmitate is the salt and ester of palmitic acid.
A herein relevant synonyms name may e.g. be palmitoate.
Beside apomorphine hydrochloride, above discussed WO2015/197839A1 and W02004/082630A2 provide a list of other possible suitable pharmaceutically acceptable salts – palmitic acid (or synonyms like palmitate or palmitoate) is not mentioned in the lists of these two WO documents.
As discussed in the review article of Schultheiss et al. (“Pharmaceutical Cocrystals and Their Physicochemical Properties”; Crystal Growth & Design, Vol. 9, No. 6, 2009, p. 2950-2967) – solid-state chemists call upon a variety of different strategies when attempting to alter the chemical and physical solid-state properties of active pharmaceutical ingredients (APIs), namely, the formation of salts, polymorphs, hydrates, solvates, and cocrystals.
Salt formation is one of the primary solid-state approaches used to modify the physical properties of APIs, and it is estimated that over half of the medicines on the market are administered as salts. However, a limitation within this approach is that the API must possess a suitable (basic or acidic)
ionizable site. In comparison, cocrystals (multicomponent assemblies held together by freely reversible, noncovalent interactions) offer a different pathway, where any API regardless of acidic, basic, or ionizable groups, could potentially be cocrystallized.
Above discussed WO02/39879A2 also provides a long list of suitable pharmaceutically acceptable salts and mentions palmitoate (see page 5, line 16).
However, in all herein relevant experimental work of this WO document was used apomorphine hydrochloride and a palmitic acid based salt is simply mentioned in a list – i.e. a palmitic acid based salt is not a preferred salt.
Alternatively expressed, by reading this WO document the skilled person has in practice no motivation to use any other solid form than apomorphine-HCI – one reason for this is that apomor-phine-HCI is used in all herein relevant experimental work of this WO document.
PATENT
WO2018130685
claiming synergistic combination comprising antimetabolite antineoplastic agent (eg cytarabine ) and type 1 serotonin receptor antagonist (5-HTR1) (eg apomorphine ), useful for treating cancer.
SYN
SYN

PAPER
- Small, L. et al.: J. Org. Chem. (JOCEAH) 5, 334 (1940)
References
- ^ Jump up to:a b Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A (November 2002). “Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes”. The Journal of Pharmacology and Experimental Therapeutics. 303 (2): 791–804. doi:10.1124/jpet.102.039867. PMID 12388666.
- ^ Guardia J, Casas M, Prat G, Trujols J, Segura L, Sánchez-Turet M (October 2002). “The apomorphine test: a biological marker for heroin dependence disorder?”. Addiction Biology. 7 (4): 421–6. doi:10.1080/1355621021000006206. PMID 14578019.
- ^ Jump up to:a b Dent JY (1949). “Apomorphine Treatment of Addiction”. British Journal of Addiction to Alcohol & Other Drugs. 46 (1): 15–28. doi:10.1111/j.1360-0443.1949.tb04502.x.
- ^ “Apomorphine Uses, Side Effects & Warnings”. Drugs.com. Retrieved 27 February2018.
- ^ Jump up to:a b c d e f Clayton BD, Willihnganz M (2016). Basic Pharmacology for Nurses – E-Book. Elsevier Health Sciences. pp. 210–211. ISBN 978-0-323-37697-6.
- ^ Jump up to:a b c d e f g h i j k l m “Apomorphine Hydrochloride Monograph for Professionals”. Drugs.com. Retrieved 26 February 2018.
- ^ Jump up to:a b c Chaudhuri KR, Clough C (February 1998). “Subcutaneous apomorphine in Parkinson’s disease”. BMJ. 316 (7132): 641. doi:10.1136/bmj.316.7132.641. PMC 1112674. PMID 9522772.
- ^ Jump up to:a b Schapira AH, Olanow CW (2005). Principles of Treatment in Parkinson’s Disease(illustrated ed.). Elsevier Health Sciences. p. 35. ISBN 978-0-7506-5428-9.
- ^ Dressler D (June 2005). “[Apomorphine in the treatment of Parkinson’s Disease]”. Der Nervenarzt (in German). 76 (6): 681–9. doi:10.1007/s00115-004-1830-4. PMID 15592807.
- ^ Jump up to:a b c Youdim MB, Gassen M, Gross A, Mandel S, Grünblatt E (2000). “Iron chelating, antioxidant and cytoprotective properties of dopamine receptor agonist; apomorphine”. In Mizuno Y, Calne D, Horowski R, Poewe W, Riederer P, Youdim M (eds.). Advances in Research on Neurodegeneration. 7 (illustrated ed.). Springer Science & Business Media. pp. 83–96. ISBN 978-3-211-83485-5.
- ^ “Apomorphine”. Medline Plus. US National Library of Medicine. 15 June 2017. Retrieved 26 February 2018.
- ^ Jump up to:a b Porst H, Buvat J (2008). Standard Practice in Sexual Medicine. John Wiley & Sons. p. 77. ISBN 978-1-4051-7872-3.
- ^ Ptácek R, Kuzelová H, Stefano GB (September 2011). “Dopamine D4 receptor gene DRD4 and its association with psychiatric disorders”. Medical Science Monitor. 17 (9): RA215–20. doi:10.12659/MSM.881925. PMC 3560519. PMID 21873960.
- ^ Stacy M, Silver D (2008). “Apomorphine for the acute treatment of “off” episodes in Parkinson’s disease”. Parkinsonism & Related Disorders. 14 (2): 85–92. doi:10.1016/j.parkreldis.2007.07.016. PMID 18083605.
- ^ Jump up to:a b c d e f g U.S. National Library of Medicine. “Apomorphine”. PubChem. Retrieved 26 February 2018.
- ^ Lewitt P, Oertel WH (1999). Parkinsons’s Disease: The Treatment Options. CRC Press. p. 22. ISBN 978-1-85317-379-0.
- ^ Rektorova I, Sedlackova S, Telecka S, Hlubocky A, Rektor I (2008). “Dorsolateral prefrontal cortex: a possible target for modulating dyskinesias in Parkinson’s disease by repetitive transcranial magnetic stimulation”. International Journal of Biomedical Imaging. 2008: 372125. doi:10.1155/2008/372125. PMC 2233877. PMID 18274665.
- ^ Jump up to:a b Galvez-Jimenez N (2013). Scientific Basis for the Treatment of Parkinson’s Disease, Second Edition. CRC Press. p. 195. ISBN 978-0-203-33776-9.
- ^ Jump up to:a b Iversen L (2012). Biogenic Amine Receptors. Springer Science & Business Media. p. 238. ISBN 978-1-4684-8514-1.
- ^ Jump up to:a b c Advances in Pharmacology and Chemotherapy, Volume 15. Silvio Garattini, A. Goldin, F. Hawking, Irwin J. Kopin. Academic Press. 1978. pp. 27, 93, 96. ISBN 978-0-08-058106-4.
- ^ Ohta M, Mizuta I, Ohta K, Nishimura M, Mizuta E, Hayashi K, Kuno S (May 2000). “Apomorphine up-regulates NGF and GDNF synthesis in cultured mouse astrocytes”. Biochemical and Biophysical Research Communications. 272 (1): 18–22. doi:10.1006/bbrc.2000.2732. PMID 10872797.
- ^ McGeary JE, Gurel V, Knopik VS, Spaulding J, McMichael J (October 2011). “Effects of nerve growth factor (NGF), fluoxetine, and amitriptyline on gene expression profiles in rat brain”. Neuropeptides. 45 (5): 317–22. doi:10.1016/j.npep.2011.06.002. PMID 21820738.
- ^ Heberlein A, Muschler M, Frieling H, Behr M, Eberlein C, Wilhelm J, Gröschl M, Kornhuber J, Bleich S, Hillemacher T (May 2013). “Epigenetic down regulation of nerve growth factor during alcohol withdrawal”. Addiction Biology. 18 (3): 508–10. doi:10.1111/j.1369-1600.2010.00307.x. PMID 21392176.
- ^ Riviere JE, Papich MG (2009). Veterinary Pharmacology and Therapeutics. John Wiley & Sons. p. 318. ISBN 978-0-8138-2061-3.
- ^ Roth BL, Driscol J (12 January 2011). “PDSP Ki Database”. Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 1 July 2014. Note: Values for humans are used. If there is more than one value listed for humans, their average is used.
- ^ Newman-Tancredi A, Cussac D, Audinot V, Nicolas JP, De Ceuninck F, Boutin JA, Millan MJ (November 2002). “Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. II. Agonist and antagonist properties at subtypes of dopamine D(2)-like receptor and alpha(1)/alpha(2)-adrenoceptor”. The Journal of Pharmacology and Experimental Therapeutics. 303 (2): 805–14. doi:10.1124/jpet.102.039875. PMID 12388667.
- ^ Newman-Tancredi A, Cussac D, Quentric Y, Touzard M, Verrièle L, Carpentier N, Millan MJ (November 2002). “Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. III. Agonist and antagonist properties at serotonin, 5-HT(1) and 5-HT(2), receptor subtypes”. The Journal of Pharmacology and Experimental Therapeutics. 303 (2): 815–22. doi:10.1124/jpet.102.039883. PMID 12388668.
- ^ Hsieh GC, Hollingsworth PR, Martino B, Chang R, Terranova MA, O’Neill AB, Lynch JJ, Moreland RB, Donnelly-Roberts DL, Kolasa T, Mikusa JP, McVey JM, Marsh KC, Sullivan JP, Brioni JD (January 2004). “Central mechanisms regulating penile erection in conscious rats: the dopaminergic systems related to the proerectile effect of apomorphine”. The Journal of Pharmacology and Experimental Therapeutics. 308 (1): 330–8. doi:10.1124/jpet.103.057455. PMID 14569075.
- ^ Lewis, R.J. Sr. (2004). Sax’s Dangerous Properties of Industrial Materials (11 ed.). Wiley, John & Sons, Incorporated. p. 287. ISBN 978-0471476627.
- ^ Jump up to:a b Narayanasamy Gurusamy. “Process for making apomorphine and apocodeine”.
- ^ Bentley, K.W. (2014-04-24). The Isoquinoline Alkaloids: A Course in Organic Chemistry. Elsevier, 2014. pp. 118–120. ISBN 978-1483152233.
- ^ Poklis JL, Mulder HA, Halquist MS, Wolf CE, Poklis A, Peace MR (July 2017). “The Blue Lotus Flower (Nymphea caerulea) Resin Used in a New Type of Electronic Cigarette, the Re-Buildable Dripping Atomizer”. Journal of Psychoactive Drugs. 49 (3): 175–181. doi:10.1080/02791072.2017.1290304. PMC 5638439. PMID 28266899.
- ^ Bertol E, Fineschi V, Karch SB, Mari F, Riezzo I (February 2004). “Nymphaea cults in ancient Egypt and the New World: a lesson in empirical pharmacology”. Journal of the Royal Society of Medicine. 97 (2): 84–5. doi:10.1177/014107680409700214. PMC 1079300. PMID 14749409.
- ^ Taba P, Lees A, Stern G (2013). “Erich Harnack (1852-1915) and a short history of apomorphine”. European Neurology. 69 (6): 321–4. doi:10.1159/000346762. PMID 23549143.
- ^ Gee S (1869). “On the action of a new organic base, apomorphia”. Transactions of the Clinical Society of London. 2: 166–169.
- ^ Feser J (1873). “Die in neuester Zeit in Anwendung gekommen Arzneimittel: 1. Apomorphinum hydrochloratum”. Z Prakt Veterinairwiss: 302–306.
- ^ Tompkins J (1899). “Apomorphine in Acute Alcoholic Delirium”. Medical Record.
- ^ “Apomorphine as a hypnotic”. The Lancet. 155 (3998): 1083. 1900. doi:10.1016/s0140-6736(01)70565-x.
- ^ Douglas CJ (1899). “The withdrawal of alcohol in delirium tremens”. The New York Medical Journal: 626.
- ^ Hare, Francis (1912). On alcoholism; its clinical aspects and treatment. London: Churchill.
- ^ Benes FM (January 2001). “Carlsson and the discovery of dopamine”. Trends in Pharmacological Sciences. 22 (1): 46–7. doi:10.1016/S0165-6147(00)01607-2. PMID 11165672.
- ^ Ernst AM (May 1965). “Relation between the action of dopamine and apomorphine and their O-methylated derivatives upon the CNS”. Psychopharmacologia. 7 (6): 391–9. doi:10.1007/BF00402361. PMID 5831877.
- ^ Moynihan NH (1965). “The Treatment of Alcoholism in General Practice”. Practitioner: 223–7.
- ^ Carlsson C, Johansson PR, Gullberg B (May 1977). “A double-blind cross-over study: apomorphine/placebo in chronic alcoholics”. International Journal of Clinical Pharmacology and Biopharmacy. 15 (5): 211–3. PMID 326687.
- ^ Halvorsen KA, Martensen-Larsen O (April 1978). “Apomorphine revived: fortified, prolonged, and improved therapeutical effect”. The International Journal of the Addictions. 13 (3): 475–84. doi:10.3109/10826087809045262. PMID 352969.
- ^ Jensen SB, Christoffersen CB, Noerregaard A (December 1977). “Apomorphine in outpatient treatment of alcohol intoxication and abstinence: a double-blind study”. The British Journal of Addiction to Alcohol and Other Drugs. 72 (4): 325–30. doi:10.1111/j.1360-0443.1977.tb00699.x. PMID 341937.
- ^ Schlatter EK, Lal S (June 1972). “Treatment of alcoholism with Dent’s oral apomorphine method”. Quarterly Journal of Studies on Alcohol. 33 (2): 430–6. PMID 5033142.
- ^ Weil E (1884). “De l’apomorphine dans certain troubles nerveux”. Lyon Med (in French). 48: 411–419.
- ^ Schwab RS, Amador LV, Lettvin JY (1951). “Apomorphine in Parkinson’s disease”. Transactions of the American Neurological Association. 56: 251–3. PMID 14913646.
- ^ Cotzias GC, Papavasiliou PS, Fehling C, Kaufman B, Mena I (January 1970). “Similarities between neurologic effects of L-dopa and of apomorphine”. The New England Journal of Medicine. 282 (1): 31–3. doi:10.1056/NEJM197001012820107. PMID 4901383.
- ^ Corsini GU, Del Zompo M, Gessa GL, Mangoni A (May 1979). “Therapeutic efficacy of apomorphine combined with an extracerebral inhibitor of dopamine receptors in Parkinson’s disease”. Lancet. 1 (8123): 954–6. doi:10.1016/S0140-6736(79)91725-2. PMID 87620.
- ^ Stibe CM, Kempster P, Lees AJ & Stern GM (1988). “Subcutaneous apomorphine in parkinsonian on-off oscillations”. Lancet. 331 (8582): 403–406. doi:10.1016/S0140-6736(88)91193-2.
- ^ Ban TA (2008). Conditioning behavior and psychiatry. New Brunswick [N.J.]: AldineTransaction. ISBN 978-0-202-36235-9. OCLC 191318001.
- ^ Raikhel EA (2016). Governing habits : treating alcoholism in the post-Soviet clinic. Ithaca. ISBN 9781501703133. OCLC 965905763.
- ^ Lemere F, Voegtlin WL (June 1950). “An evaluation of the aversion treatment of alcoholism”. Quarterly Journal of Studies on Alcohol. 11 (2): 199–204. PMID 15424345.
- ^ Dent JY (1934-10-01). “Apomorphine in the Treatment of Anxiety States, with Especial Reference to Alcoholism*”. British Journal of Inebriety. 32 (2): 65–88. doi:10.1111/j.1360-0443.1934.tb05016.x. ISSN 1360-0443.
- ^ De Morsier G, Feldmann H (1952). “[Apomorphine therapy of alcoholism; report of 500 cases]”. Schweizer Archiv für Neurologie und Psychiatrie. Archives Suisses de Neurologie et de Psychiatrie. Archivio Svizzero di Neurologia e Psichiatria. 70 (2): 434–40. PMID 13075975.
- ^ “Gay injustice ‘was widespread‘“. 2009-09-12. Retrieved 2018-01-24.
- ^ Birmingham, Jed (2 November 2009). “William Burroughs and the History of Heroin”. RealityStudio.
- ^ Morales-Rosado JA, Cousin MA, Ebbert JO, Klee EW (December 2015). “A Critical Review of Repurposing Apomorphine for Smoking Cessation”. Assay and Drug Development Technologies. 13 (10): 612–22. doi:10.1089/adt.2015.680. PMID 26690764.
- ^ “Apomorphine – A forgotten treatment for alcoholism”. apomorphine.info. Retrieved 2018-01-24.
- ^ IsHak, Waguih William (2017). The Textbook of Clinical Sexual Medicine. Springer. p. 388. ISBN 978-3-319-52539-6.
- ^ “Abbott Withdraws Application for an Impotence Pill”. Bloomberg News via The New York Times. 1 July 2000.
- ^ Jump up to:a b Lashuel HA, Hartley DM, Balakhaneh D, Aggarwal A, Teichberg S, Callaway DJ (November 2002). “New class of inhibitors of amyloid-beta fibril formation. Implications for the mechanism of pathogenesis in Alzheimer’s disease”. The Journal of Biological Chemistry. 277 (45): 42881–90. doi:10.1074/jbc.M206593200. PMID 12167652.
- ^ Himeno E, Ohyagi Y, Ma L, Nakamura N, Miyoshi K, Sakae N, Motomura K, Soejima N, Yamasaki R, Hashimoto T, Tabira T, LaFerla FM, Kira J (February 2011). “Apomorphine treatment in Alzheimer mice promoting amyloid-β degradation” (PDF). Annals of Neurology. 69 (2): 248–56. doi:10.1002/ana.22319. PMID 21387370.
- ^ Jump up to:a b c d e Bill RL (2016). Clinical Pharmacology and Therapeutics for Veterinary Technicians – E-Book. Elsevier Health Sciences. p. 94. ISBN 978-0-323-44402-6.
- ^ Jump up to:a b Khan SN, Hooser SB (2012). Common Toxicologic Issues in Small Animals, an Issue of Veterinary Clinics: Small Animal Practice – E-Book. Elsevier Health Sciences. p. 310. ISBN 978-1-4557-4325-4.
- ^ Plumb, Donald C. (2011). “Apomorphine”. Plumb’s Veterinary Drug Handbook (7th ed.). Stockholm, Wisconsin: Wiley. pp. 77–79. ISBN 978-0-470-95964-0.
- ^ Peterson ME, Talcott PA (2006). Small Animal Toxicology. Elsevier Health Sciences. p. 131. ISBN 978-0-7216-0639-2.
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Apokyn |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604020 |
| Pregnancy category |
|
| Routes of administration |
SQ |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% following injection |
| Protein binding | ~50% |
| Metabolism | Hepatic, phase II |
| Onset of action | 10–20 min |
| Elimination half-life | 40 minutes |
| Duration of action | 60–90 min |
| Excretion | Hepatic |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.327 |
| Chemical and physical data | |
| Formula | C17H17NO2 |
| Molar mass | 267.322 g/mol g·mol−1 |
| 3D model (JSmol) | |
Apomorphine
-
- ATC:N04BC07
- Use:emetic, erectile dysfunction
- Chemical name:(R)-5,6,6a,7-tetrahydro-6-methyl-4H-dibenzo[de,g]quinoline-10,11-diol
- Formula:C17H17NO2
- MW:267.33 g/mol
- CAS-RN:58-00-4
- InChI Key:VMWNQDUVQKEIOC-UHFFFAOYSA-N
- InChI:InChI=1S/C17H17NO2/c1-18-8-7-10-3-2-4-12-15(10)13(18)9-11-5-6-14(19)17(20)16(11)12/h2-6,13,19-20H,7-9H2,1H3
- EINECS:200-360-0
- LD50:56 mg/kg (M, i.v.); >100 mg/kg (M, p.o.)
///////////// LK-01, LK 01 , LK01, Apomorphine
CN1CCC2=C3C1CC4=C(C3=CC=C2)C(=C(C=C4)O)O.CN1CCC2=C3C1CC4=C(C3=CC=C2)C(=C(C=C4)O)O.O.Cl.Cl



