Image may be NSFW.
Clik here to view.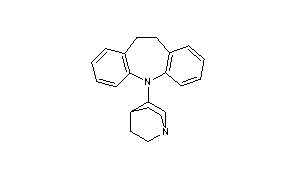
Quinupramine
キヌプラミン
- 5-(1-azabicyclo[2.2.2]oct-3-yl)-10,11-dihydro-5H-dibenz[b,f]azepine
- Formula:C21H24N2
- MW:304.44 g/mol
- CAS:31721-17-2
Quinupramine (brand names Kevopril, Kinupril, Adeprim, Quinuprine) is a tricyclic antidepressant (TCA) used in Europe for the treatment of depression.[1][2]
Pharmacologically, quinupramine acts in vitro as a strong muscarinic acetylcholine receptor antagonist (anticholinergic) and H1 receptorantagonist (antihistamine), moderate 5-HT2 receptor antagonist, and weak serotonin and norepinephrine reuptake inhibitor.[3] It has negligible affinity for the α1-adrenergic, α2-adrenergic, β-adrenergic, or D2 receptor.[3]
Clinically, quinupramine is reported to be stimulating similarly to imipramine, desipramine, and demexiptiline.[4] It can be inferred that its in vivo metabolites may have stronger effects on the reuptake of norepinephrine and/or serotonin than quinupramine itself
SYN
Image may be NSFW.
Clik here to view.
References
- ^ Swiss Pharmaceutical Society (2000). Index Nominum 2000: International Drug Directory (Book with CD-ROM). Boca Raton: Medpharm Scientific Publishers. p. 908. ISBN 3-88763-075-0.
- ^ José Miguel Vela; Helmut Buschmann; Jörg Holenz; Antonio Párraga; Antoni Torrens (2007). Antidepressants, Antipsychotics, Anxiolytics: From Chemistry and Pharmacology to Clinical Application. Weinheim: Wiley-VCH. p. 248. ISBN 978-3-527-31058-6.
- ^ Jump up to:a b Sakamoto H, Yokoyama N, Kohno S, Ohata K (December 1984). “Receptor binding profile of quinupramine, a new tricyclic antidepressant”. Japanese Journal of Pharmacology. 36 (4): 455–60. doi:10.1254/jjp.36.455. PMID 6098759.
- ^ Kent, Angela; M. Billiard (2003). Sleep: physiology, investigations, and medicine. New York: Kluwer Academic/Plenum. p. 233. ISBN 0-306-47406-9.
-
- DOS 2 030 492 (Sogeras; appl. 20.6.1970; GB-prior. 20.6.1969).
- GB 1 252 320 (Sogeras; valid from 29.5.1970; prior. 20.6.1969).
| Image may be NSFW. Clik here to view. 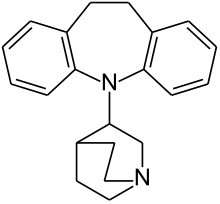 |
|
| Clinical data | |
|---|---|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 33 hours |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ECHA InfoCard | 100.046.149 Image may be NSFW. Clik here to view. |
| Chemical and physical data | |
| Formula | C21H24N2 |
| Molar mass | 304.43 g/mol g·mol−1 |
//////////////Quinupramine, キヌプラミン