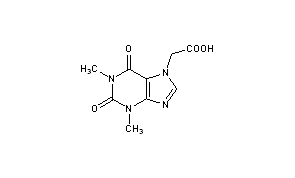
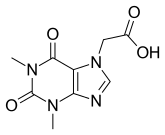
Acefylline
- Molecular FormulaC9H10N4O4
- Average mass238.200 Da
(1,3-Dimethyl-2,6-dioxo-1,2,3,6-tetrahydro-7H-purin-7-yl)acetic acid
1,2,3,6-Tetrahydro-1,3-dimethyl-2,6-dioxo-7H-purine-7-acetic Acid
1,3-Dimethylxanthine-7-acetic acid
211-490-2 [EINECS]
652-37-9 [RN]
7-(Carboxymethyl)theophylline
7H-Purine-7-acetic acid, 1,2,3,6-tetrahydro-1,3-dimethyl-2,6-dioxo-
CAS Registry Number: 652-37-9
CAS Name: 1,2,3,6-Tetrahydro-1,3-dimethyl-2,6-dioxopurine-7-acetic acid
Additional Names: carboxymethyltheophylline; 7-theophyllineacetic acid
Molecular Formula: C9H10N4O4
Molecular Weight: 238.20
Percent Composition: C 45.38%, H 4.23%, N 23.52%, O 26.87%
Literature References: Prepn: DE 352980 (1922 to E. Merck); Frdl. 14, 1320; S. M. Ride et al., Pharmazie 32, 672 (1977). Prepn of salts: J. Baisse, Bull. Soc. Chim. Fr. 1949, 769; M. Milletti, F. Virgili, Chimica 6, 394 (1951), C.A. 46, 8615h (1952). GC determn in urine: J. Zuidema, H. Hilbers, J. Chromatogr. 182, 445 (1980). HPLC determn in serum and pharmacokinetics: S. Sved et al.,Biopharm. Drug Dispos. 2, 177 (1981).
Properties: Crystals from water, mp 271°.
Melting point: mp 271°
Derivative Type: Sodium salt
CAS Registry Number: 837-27-4
Molecular Formula: C9H9N4NaO4
Molecular Weight: 260.18
Percent Composition: C 41.55%, H 3.49%, N 21.53%, Na 8.84%, O 24.60%
Properties: Silky needles, mp >300°.
Melting point: mp >300°
Derivative Type: Compd with piperazine
Additional Names: Acefylline piperazine; acepifylline
Trademarks: Dynaphylline (Welcker-Lyster); Etaphylline (Delalande); Etafillina (Delalande)
Properties: Undefined mixture of the 1:1 and 2:1 salts; contains 75-78% theophylline acetic acid and 22-25% anhydrous piperazine.
Therap-Cat: Bronchodilator.
Keywords: Bronchodilator; Xanthine Derivatives.
Acefylline (INN),[1] also known as acetyloxytheophylline, is a stimulant drug of the xanthine chemical class. It acts as an adenosine receptor antagonist. It is combined with diphenhydramine in the pharmaceutical preparation etanautine to help offset diphenhydramine induced drowsiness.[2]
Synthesis
DE 352980 (1922 to E. Merck); Frdl. 14, 1320; S. M. Ride et al., Pharmazie 32, 672 (1977).

Acefylline
- Use:cardiotonic, diuretic, antispasmodic, bronchodilator
- Chemical name:1,2,3,6-tetrahydro-1,3-dimethyl-2,6-dioxo-7H-purine-7-acetic acid
- Formula:C9H10N4O4
- MW:238.20 g/mol
- CAS-RN:652-37-9
- EINECS:211-490-2
- LD50:1180 mg/kg (M, i.p.); 2733 mg/kg (M, p.o.)
Acepifylline
- Use:
- Chemical name:1,2,3,6-tetrahydro-1,3-dimethyl-2,6-dioxo-7H-purine-7-acetic acid compd. with piperazine
- Formula:C9H10N4O4 • xC4H10N2
- MW:unspecified
- CAS-RN:18833-13-1
- EINECS:242-614-3
Acefylline heptaminol
- Use:
- Chemical name:1,2,3,6-tetrahydro-1,3-dimethyl-2,6-dioxo-7H-purine-7-acetic acid compd. with 6-amino-2-methyl-2-heptaminol (1:1)
- Formula:C9H10N4O3 • C8H19NO
- MW:367.45 g/mol
- CAS-RN:59989-20-7
- EINECS:262-012-4
References
- ^ “International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names (Rec. INN): List 21” (PDF). World Health Organization. Retrieved 29 December 2016.
- ^ Zuidema, Jan. (1978). “Biofarmaceutische en farmacokinetische aspecten van theofylline en acefylline”. Thesis (doctoral)–Universiteit van Amsterdam. References
Baisse, J.: Bull. Soc. Chim. Fr. (BSCFAS) 1949, 769.
DE 352 980 (E. Merck; 1922).
 |
|
 |
|
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| CAS Number | |
| PubChemCID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| ECHA InfoCard | 100.010.447  |
| Chemical and physical data | |
| Formula | C9H10N4O4 |
| Molar mass | 238.20 g/mol g·mol−1 |
| 3D model (JSmol) | |
////////Acefylline

