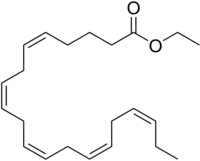
Icosapent ethyl
330.5042 , C22H34O2
cas 86227-47-6 / 73310-10-8
ethyl (5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaenoate
Ethyl eicosapentaenoic acid
イコサペント酸エチル
- 5,8,11,14,17-Eicosapentaenoic acid, ethyl ester, (all-Z)-
- (5Z,8Z,11Z,14Z,17Z)-5,8,11,14,17-Eicosapentaenoic acid ethyl ester
- (all-Z)-5,8,11,14,17-Eicosapentaenoic acid ethyl ester
- AMR 101
- C20:5 n-3 Ethyl ester
- Epadel
- Epadel S 300
- Ethyl (5Z,8Z,11Z,14Z,17Z)-eicosa-5,8,11,14,17-pentaenoate
- Ethyl all-Z-5,8,11,14,17-eicosapentanenoate
- Ethyl all-cis-5,8,11,14,17-eicosapentaenoate
- Ethyl eicosapentaenoate
- Ethyl icosapentate
- Icosapent ethyl
- Incromega EPA
- Timnodonic acid ethyl ester
- Vascepa
- cis-Eicosapentaenoic acid ethyl ester
(all-Z)-5,8,11,14,17-Eicosapentaenoic acid ethyl ester; Ethyl all-cis-5,8,11,14,17-eicosapentaenoate;Timnodonic acid ethyl ester; cis-Eicosapentaenoic acid ethyl ester; Ethyl (5Z,8Z,11Z,14Z,17Z)-eicosa-5,8,11,14,17-pentaenoate; Epadel; Icosapent; EPA ethyl ester; E-EPA; Ethyl eicosapentaenoate; OMEGA-3 ACIDS ETHYL ESTER; EPA-E;
AMARIN PHARMACEUTICALS IRELAND LTD
AMR 101 / AMR-101 / AMR101
Icosapent ethyl or ethyl eicosapentaenoic acid is a synthetic derivative of the omega-3 fatty acid eicosapentaenoic acid (EPA). It is used as adjunct therapy for severe hypertriglyceridemia (TG levels > 500 mg/dL). FDA approved on July 26, 2012.
In 2000, Amarin licensed exclusive U.S. rights to icosapent ethyl ester from the Scottish company Laxdale, and acquired the company in July 2004. In 2015, the product was licensed to Eddingpharm by Amarin for the development and commercialization in China, Hong Kong and Taiwan. Fast-track status has been granted in the U.S. for the treatment of HD. Orphan drug designation was assigned to the compound for this indication in both the U.S. and E.U.
fda
IND 107616 was submitted on 25 March 2010 for the indication of severe hypertriglyceridemia; Epanova had been previously investigated for the treatment of Crohn’s Disease under IND in the Division of Gastroenterology Products. An end-of-phase 2 (EOP2) meeting was held on 02 June 2010. Regarding the indication under consideration at this time, a special protocol assessment (SPA) for the single phase 3 trial OM-EPA-003 (also known as “EVOLVE”) was submitted 02 July 2010 and ultimately agreed upon, after amendments, on 22 October 2010. On 25 April 2012, the applicant proposed an alternative to conducting a thorough QTc study by assessing ECGs recorded during OM-EPA-003; this was found acceptable. A clinical pre-NDA meeting was held on 14 November 2012. The nonclinical development strategy was found reasonable. A clinical package containing OM-EPA-003 (pivotal) and OMEPA-004 (a 6-week phase 3 trial , with long-term safety supported by data from the former Crohn’s disease program (“EPIC” trials), was found adequate for submission. Agreement was reached regarding the clinical pharmacology portion of the submission. Details regarding data pooling for the Integrated Summary of Safety (ISS) were found acceptable
from the former Crohn’s disease program (“EPIC” trials), was found adequate for submission. Agreement was reached regarding the clinical pharmacology portion of the submission. Details regarding data pooling for the Integrated Summary of Safety (ISS) were found acceptable
CMC Drug Substance & Drug Product Chemistry, manufacturing, and controls data related to both the drug substance (omega-3- carboxylic acids) and drug product (Epanova Capsules 1 g) are detailed in the review by Martin Haber, PhD, and Xavier Ysern, PhD. They recommend the NDA for approval. There are no pending CMC issues. The drug substance at sites in Nova Scotia and Prince Edward Island, Canada, from crude fish oil obtained from fish It is a complex mixture of PUFAs, predominantly the omega-3 acids EPA (55%), DHA (20%), and docosapentaenoic acid %). It consistently contains omega-3 and omega-6 PUFA components: total omega-3 fatty acids are limited to not less than % and total omega-6 fatty acids are limited to not more than %. The drug substance also contains 0.3% (m/m) α-tocopherol as . During purification, . Environmental pollutants (heavy metals, pesticides, are controlled by specific tests on the drug substance . Drug substance specifications include tests for acid value, saponification value, ester value, peroxide value, p-anisidine value, total oxidation value, cholesterol, oligomers, , fatty acid composition (PUFAs, EPA, DHA, DPA, total omega-3 fatty acids, total omega-6 fatty acids, other polyunsaturated fatty acids, As described in the review by Drs. Haber and Ysern, the qualitative identify of the drug substance was developed by examining consistencies of peak patterns across 21 discrete lots: there are omega-3 and omega-6 PUFA peaks consistently present in the GC chromatograms (although not necessarily always above the limit of quantitation), which can be used to establish the fingerprint identity of omega-3-carboxylic acids . The quantitative fatty acid composition is given in the table below, excerpted from p. 25 of their review:
Ethyl eicosapentaenoic acid (E-EPA, icosapent ethyl) is a derivative of the omega-3 fatty acid eicosapentaenoic acid (EPA) that is used in combination with changes in diet to lower triglyceride levels in adults with severe (≥ 500 mg/dL) hypertriglyceridemia. This was the second class of fish oil-based drug to be approved for use as a drug and was approved by the FDA in 2012. These fish oil drugs are similar to fish oil dietary supplements but the ingredients are better controlled and have been tested in clinical trials.
The company that developed this drug, Amarin Corporation, challenged the FDA’s ability to limit its ability to market the drug for off-label use and won its case on appeal in 2012, changing the way the FDA regulates pharmaceutical marketing.
Medical use
E-EPA is used in addition to changes in diet to reduce triglyceride levels in adults with severe (≥ 500 mg/dL) hypertriglyceridemia.[1]
Intake of large doses (2.0 to 4.0 g/day) of long-chain omega-3 fatty acids as prescription drugs or dietary supplements are generally required to achieve significant (> 15%) lowering of triglycerides, and at those doses the effects can be significant (from 20% to 35% and even up to 45% in individuals with levels greater that 500 mg/dL). It appears that both eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) lower triglycerides, however, DHA alone appears to raise low-density lipoprotein (the variant which drives atherosclerosis; sometimes very inaccurately called: “bad cholesterol”) and LDL-C values (always only a calculated estimate; not measured by labs from person’s blood sample for technical and cost reasons), whilst EPA alone, does not and instead lowers the parameters aforementioned.[2]
Other fish-oil based drugs
There are other omega-3 fish oil based drugs on the market that have similar uses and mechanisms of action.[3]
- Omega-3 acid ethyl esters (brand names Omarcor or Lovaza,[4] Omtryg,[5] and as of March 2016, four generic versions.[6]
- Omega-3 carboxylic acids (Epanova)[7]
Dietary supplements
There are many fish oil dietary supplements on the market.[8] There appears to be little difference in effect between dietary supplements and prescription forms of omega-3 fatty acids, but EPA and DHA ethyl esters (prescription forms) work less well when taken on an empty stomach or with a low-fat meal.[2] The ingredients of dietary supplements are not as carefully controlled as prescription products and have not been fixed and tested in clinical trials, as prescription drugs have,[9] and the prescription forms are more concentrated, requiring fewer capsules to be taken and increasing the likelihood of compliance.[8]
Side effects
Special caution should be taken with people who have with fish and shellfish allergies.[1] In addition, as with other omega-3 fatty acids, taking E-EPA puts people who are on anticoagulants at risk for prolonged bleeding time.[1][2] The most commonly reported side effect in clinical trials has been joint pain; some people also reported pain in their mouth or throat.[1] E-EPA has not been tested in pregnant women is rated pregnancy category C; it is excreted in breast milk and the effects on infants are not known.[1]
Pharmacology
After ingestion, E-EPA is metabolized to EPA. EPA is absorbed in the small intestine and enters circulation. Peak plasma concentration occurs about 5 hours after ingestion and the half-life is about 89 hours. EPA is metabolized mostly in the liver like other dietary fatty acids.[1]
Mechanism of action
EPA, the active metabolite of E-EPA, like other omega-3 fatty acid based drugs, appears to reduce production of triglycerides in the liver, and to enhance clearance of triglycerides from circulating very low-density lipoprotein (VLDL) particles; the way it does that is not clear, but potential mechanisms include increased breakdown of fatty acids; inhibition of diglyceride acyltransferase which is involved in biosynthesis of triglycerides in the liver; and increased activity of lipoprotein lipase in blood.[1][3]
Physical and chemical properties[edit]
E-EPA is an ethyl ester of eicosapentaenoic acid, which is an omega-3 fatty acid.[1]
History
In July 2012, the US Food and Drug Administration approved E-EPA for severe hypertriglyceridemia as an adjunct to dietary measures; Amarin Corporation had developed the drug.[10]
E-EPA was the second fish-oil drug to be approved, after omega-3 acid ethyl esters (GlaxoSmithKline‘s Lovaza which was approved in 2004[11]) and sales were not as robust as Amarin had hoped. The labels for the two drugs were similar, but doctors prescribed Lovaza for people who had triglycerides lower than 500 mg/dL based on some clinical evidence. Amarin wanted to actively market E-EPA for that population as well which would have greatly expanded its revenue, and applied to the FDA for permission to do so in 2013, which the FDA denied.[12] In response, in May 2015 Amarin sued the FDA for infringing its First Amendment rights,[13] and in August 2015 a judge ruled that the FDA could not “prohibit the truthful promotion of a drug for unapproved uses because doing so would violate the protection of free speech.”[14] The ruling left open the question of what the FDA would allow Amarin to say about E-EPA, and in March 2016 the FDA and Amarin agreed that Amarin would submit specific marketing material to the FDA for the FDA to review, and if the parties disagreed on whether the material was truthful, they would seek a judge to mediate.[15]
PAPER
https://link.springer.com/article/10.1023%2FB%3ACONC.0000039128.78645.a8
Synthesis of Fatty-Acid Ethanolamides from Linum catharticum Oils and Cololabis saira Fats
Chemistry of Natural Compounds (Translation of Khimiya Prirodnykh Soedinenii) (2004), 40, (3), 222-226
PAPER
Journal of Molecular Catalysis B: Enzymatic, 84, 173-176; 2012
https://www.sciencedirect.com/science/article/pii/S1381117712000896?via%3Dihub
STARTING MATERIAL CAS 10417-94-4
- (all-Z)-Δ5,8,11,14,17-Eicosapentaenoic acid
- (all-cis)-5,8,11,14,17-Eicosapentaenoic acid

PATENT
CN 104846023
https://patents.google.com/patent/CN104846023A/en
Example 1
[0041] A method for preparing a concentrated fish oil fatty acid glycerides, the process steps shown in Figure 1, comprising the steps of:
[0042] S11 using crude enzyme preparation of deep sea fish art: the ratio: (m m) of deep-sea fish through the machine crushed bone formation minced, weighed 600g yue meat, meat by:: water = 0 5.1 water was added seal, in the dark, under nitrogen flow, at 75 ° C cooking lh. Using NaOH to adjust pH to 8.0. Mass fraction of 2% trypsin (trypsin: food grade, Zhengzhou Hong Cheng Chemical Products Limited), in the dark, enzyme 17h at 20 ° C. After 20min by centrifugation 3000r / min, the upper layer was enzymolysis, namely crude fish oil;
[0043] S12 is prepared refined fish oil: Crude fish oil prepared in Step S11 is added a volume ratio of 0.5% phosphoric acid: degummed (crude phosphoric acid fish oil), a concentration of 70% phosphoric acid, followed by centrifugation speed of 3000 rpm / min, and then add a volume ratio of 1% deacidification NaOH, the NaOH concentration is 20%, after centrifugation, the rotational speed of 3000- rpm / min, to obtain refined fish oil;
. [0044] S13 of the refined fish oil fatty acid ethyl ester prepared by esterification process: step S12 is added to the fish oil refining prepared in mass ratio of 0.5% of sodium ethoxide, and a mass ratio of 0.5 in ethanol (ethanol: fish oil refining ), 40 ° C water bath for 1 hour, 1% (by mass) citric acid (citric acid: fish oil refining), standing layer, the upper layer and the liquid was washed with hot deionized water, standing layered repeated three times to give fatty acid ethyl ester.
. [0045] S14 of the fatty acid ethyl ester was extracted Separation: fatty acid ethyl ester obtained in step S13 is subjected to supercritical fluid extraction (extraction process of separation vessel as a rectification column I – separation kettle II), extraction conditions: a rectification column temperature 25-30-35-40 ° C, a pressure of 6 MPa rectification column, separation kettle I temperature 25 ° C, pressure in the separator tank I is 6 MPa, the temperature in the separation tank II 30-45 ° C, C0 2 flow rate of 151,711;
. [0046] S15 of the fatty acid ethyl ester after enzymatic extraction separation processing: The fatty acid ethyl ester obtained in step S14 using Penicillium expansum lipase enzyme, 4% of the amount of enzyme added,, reaction temperature 40 ° C , reaction pH of 10, speed 150 revolutions / min, hydrolysis time 4h, to obtain fatty acid glycerides.
[0047] Example 2
[0048] A process for preparing concentrated fish oil fatty acid glycerides, comprising the steps of:
. [0049] S21 using crude enzyme preparation of deep sea fish art: The procedure of Example 1 with reference to embodiment 11, wherein the cooking temperature is 85 ° C, hydrolysis temperature 25 ° C, centrifuge speed is 4000r / min;
. [0050] S22 refined fish oil preparation: The procedure of Example 1 with reference to embodiment 12; wherein, phosphate: the crude fish oil volume ratio is 1.5%, the phosphoric acid concentration of 75%; K0H: crude fish oil volume ratio of 3%, K0H the concentration of 30%, a centrifugal speed of 4000r / min;
. [0051] S23 of the refined fish oil fatty acid ethyl ester prepared by esterification process: The procedure of Example 1 with reference to embodiment 13; wherein, potassium ethoxide: refined fish oil mass ratio of 1 billion% ethanol: refined fish oil mass ratio of 2.0 , heat the water bath 60 ° C for 3 hours, and acetic acid is acetic acid: refined fish oil mass ratio of 3.0%;
. [0052] S24 was extracted to separate fatty acid ethyl ester: The procedure of Example 1 with reference to embodiment 14; wherein the extraction conditions: temperature rectification column 30-35-40-45 ° C, a pressure rectification column is 15 megabytes Pa, temperature of separation vessel I 35 ° C, pressure in the separator tank I is 8 MPa, the temperature in the separation tank II was 40 ° C, C0 2 flow rate of 171,711;
. [0053] S25 of the fatty acid ethyl ester after enzymatic extraction is carried out the separation treatment: The procedure of Example 1 with reference to embodiment 15; wherein 10% of the amount of enzyme added, reaction temperature 50 ° C, pH 8 hydrolysis, speed 300 rpm / min, hydrolysis time 12h, to obtain fatty acid glycerides.
[0054] Example 3
[0055] – Preparation Method Species of concentrated fish oil fatty acid glycerides, comprising the steps of:
. [0056] S31 using crude enzyme preparation of deep sea fish art: The procedure of Example 1 with reference to embodiment 11, wherein the cooking temperature is 90 ° C, hydrolysis temperature 35 ° C, centrifuge speed is 5000r / min;
. [0057] S32 prepared fine fish oil: The procedure of Example 1 with reference to embodiment 12; wherein, phosphate: the crude fish oil volume ratio of 3% phosphoric acid concentration of 85%; NaOH: crude fish oil volume ratio of 6% and the concentration of NaOH 50%, a centrifugal speed of 5000r / min;
. [0058] S33 of the refined fish oil fatty acid ethyl ester prepared by esterification process: The procedure of Example 1 with reference to embodiment 13; wherein, potassium ethoxide: refined fish oil mass ratio of 1.5%, ethanol: refined fish oil mass ratio of 4.0 heat treatment is 80 ° C water bath for 5 hours, citric acid and citric acid are added: refined fish oil mass ratio of 5.0%;
. [0059] S34 was extracted to separate fatty acid ethyl ester: The procedure of Example 1 with reference to embodiment 14; wherein the extraction conditions: temperature rectification column 30-35-40-45 ° C, pressure column 17 trillion Pa, I of separation vessel temperature 40 ° C, pressure in the separator tank I is 10 MPa, the temperature in the separation tank II is 45 ° C, C0 2 flow rate is? L / h;
. [0060] S35 of the fatty acid ethyl ester after enzymatic extraction separation processing: The procedure of Example 1 with reference to embodiment 15; wherein 20% of the amount of enzyme added, reaction temperature 60 ° C, a pH of 6.5 hydrolysis, speed 300 rpm / min, hydrolysis time 24h, to obtain fatty acid glycerides.
[0061] Comparative Example
[0062] S1 • obtaining crude fish: The procedure of Example 1 with reference to embodiment 11;
. [0063] S2 refined fish oil preparation: see Example 1, Step 12;
. [0064] S3 of refined fish oil fatty acid ethyl ester prepared by esterification process: Step 1, Example 13 process embodiment with reference, to obtain fatty acid ethyl ester.
PATENT
https://patents.google.com/patent/WO2014054435A1
WO 2014054435
Patent Document 2: Japanese Patent Application Laid-Open No. 7-242895
Patent Document 3: Japanese Patent No. 3005638
Non-patent literature
9 μL of the measurement sample was diluted to 1.5 mL of n-hexane, and the content ratio of each fatty acid and the content ratio of isomers were analyzed using a gas chromatography analyzer (Type 6890 GC, manufactured by Agilent Technologies) under the following conditions did. The results are expressed as mass% converted from the area of the chromatogram.
<Column condition>
Column: DB-WAX 0.25 mm × 30 m manufactured by J & W Co., column temperature: 210 ° C.
He flow rate: 1.0 ml / min, He pressure: 134 kPa
<Detection condition>
H 2 flow rate: 30 ml / min, Air flow rate : 400 ml / min
He flow rate: 10 ml / min, DET temperature: 260 ° C.
The isomer ratio in the target highly unsaturated fatty acid was obtained by the following formula.
Raw material: 1000 mL of anhydrous ethanol solution in which 50 g of sodium hydroxide was dissolved was added to 1 kg of sardine oil, mixed and stirred at 70 to 80 ° C. for 1 hour, then 500 mL of water was added and mixed well, 1 It was left standing for a while. The separated aqueous phase was removed and the oil phase was washed several times with water to neutralize the washings to give 820 g of ethyl esterified sardine oil.
As shown in Table 1, the composition of the sardine oil was 44.09% (mass%, hereinafter the same) of eicosapentaenoic acid (EPA), 1.52% of eicosatetraenoic acid (ETA), 1.52% of arachidonic acid (AA) 1.77%, docosahexaenoic acid (DHA) 6.92%. Also, the trans isomer ratio in EPA was 1.23%.
Step (1) 160 ml of n-hexane was added to 300 g of the ethyl esterified sardine oil prepared above, and the mixture was stirred well and dissolved. To this was added 500 mL of an aqueous solution containing 50% by weight of silver nitrate, and the mixture was stirred under conditions of 5 to 30 ° C. After standing, the separated n-hexane phase was removed, and the aqueous phase was recovered.
Step (2): 2000 mL of fresh n-hexane was added to the aqueous phase obtained in the step (1), and the mixture was sufficiently stirred at 50 to 69 ° C. to extract the fatty acid ethyl ester into n-hexane. After standing, the separated aqueous phase was removed and the n-hexane phase was concentrated. The crude fatty acid ethyl ester crude product contained in this n-hexane phase contained 74.54% EPA, 0.32% ETA, 0.17% AA and 14.87% DHA in total fatty acids as shown in Table 1 It was. Also, the trans isomer ratio in EPA was 0.19%.
Step (3): The n-hexane phase containing the fatty acid ethyl ester obtained in the step (2) was maintained under conditions of a top vacuum degree of 1 Pa or less and a distillation temperature of 170 to 190 ° C. using a packed tower precision distillation apparatus While performing vacuum distillation to obtain a highly purified EPA ethyl ester-containing composition in a yield of about 60%. As shown in Table 1, this EPA ethyl ester-containing composition contained 98.25% of EPA, 0.43% of ETA, 0.21% of AA, and 0.05% of DHA in total fatty acids. Also, the trans isomer ratio in EPA was 0.45%.
The yield of EPA in this example in which the steps were performed in the order of (1), (2), (3) was about 53%.
steps (1), (2) and (3) were carried out in the same manner as in Example 1 except that the step (3) was carried out while maintaining the distillation temperature of 180 to 185 ° C., EPA ethyl ester-containing composition was obtained in a yield of about 58%. As shown in Table 1, this EPA ethyl ester-containing composition contained 98.29% of EPA, 0.40% of ETA, 0.32% of AA, and 0.05% of DHA in total fatty acids. Also, the trans isomer ratio in EPA was 0.28%, and the trans isomer was extremely small.
Comparative Example 1 An
EPA ethyl ester-containing composition was obtained in the same manner as in Example 1, except that the top vacuum degree was set to 13.3 Pa (0.1 Torr) in the step (3). As shown in Table 1, the composition contained EPA content ratio as high as 97.44% in the total fatty acid, but the trans isomer ratio in EPA was high (1.37%).
EPA ethyl ester-containing composition was obtained by performing vacuum distillation (step (3)) of ethyl esterified sardine oil and then steps (1) and (2). The conditions of each step were the same as in Example 1. As shown in Table 1, this composition contained 95.05% EPA, 0.72% ETA, 0.50% AA, 0.21% DHA in total fatty acids, the trans isomer ratio in EPA was 1.55% Met. The yield of EPA in this comparative example in which the steps were carried out in the order of (3), (1) and (2) was about 31%, and the EPA yield greatly decreased as compared with Example 1.
By changing the condition of the vacuum distillation in this Comparative Example (0.5 Pa, 185 to 195 ° C.), it was possible to raise the content of EPA in the total fatty acids in the composition to 98.12%, however, The rate further declined and the trans isomer ratio in EPA was 2.01%, further increased.
step (3), the distillation temperature was 180 ° C. (Example 3), 190 ° C. (Example 4), 200 ° C. (Comparative Example 3), and the vacuum distillation time was A highly purified EPA ethyl ester-containing composition was obtained in the same manner as in Example 1 except that various changes were made and the trans isomer ratio of EPA in the composition was determined. The results are shown in Fig. 1. 1, in Examples 3 to 4 having a distillation temperature of 190 ° C. or less, the trans isomer ratio was less than 1% by mass, but in Comparative Example 3 having a distillation temperature of 200 ° C., the trans isomer The ratio exceeds 1% by mass.
References
- ^ Jump up to:a b c d e f g h Icosapent ethyl Label Last revised June 2015. Check for updates at FDA label index page here
- ^ Jump up to:a b c Jacobson TA, et al, NLA Expert Panel. National Lipid Association Recommendations for Patient-Centered Management of Dyslipidemia: Part 2. J Clin Lipidol. 2015 Nov-Dec;9(6 Suppl):S1-S122.e1. PMID 26699442 Free full text
- ^ Jump up to:a b Weintraub, HS (2014). “Overview of prescription omega-3 fatty acid products for hypertriglyceridemia”. Postgrad Med. 126: 7–18. doi:10.3810/pgm.2014.11.2828. PMID 25387209. Retrieved 20 April 2015.
- Jump up^ University of Utah Pharmacy Services (15 August 2007) “Omega-3-acid Ethyl Esters Brand Name Changed from Omacor to Lovaza”
- Jump up^ Omtryg Label Revised April 2014
- Jump up^ FDA Omega-3 acid ethyl esters products Page accessed 31 March 2016
- Jump up^ “Epanova (omega-3-carboxylic acids)”. CenterWatch. Retrieved 15 December 2014.
- ^ Jump up to:a b Ito MK. A Comparative Overview of Prescription Omega-3 Fatty Acid Products. P T. 2015 Dec;40(12):826-57. PMID 26681905 Free PMC Article PMC 4671468
- Jump up^ Sweeney MET. Hypertriglyceridemia Pharmacologic Therapy for Medscape Drugs & Diseases, Ed. Khardori R. Updated: 14 April 2015, page accessed 1 April 2016
- Jump up^ CenterWatch Vascepa (icosapent ethyl) Page accessed 31 March 2016
- Jump up^ VHA Pharmacy Benefits Management Strategic Healthcare Group and the Medical Advisory Panel. October 2005 National PBM Drug Monograph Omega-3-acid ethyl esters (Lovaza, formerly Omacor)
- Jump up^ Matthew Herper for Forbes. 17 October 2013 Why The FDA Is Right To Block Amarin’s Push To Market Fish Oil To Millions
- Jump up^ Thomas, Katie (7 May 2015). “Drugmaker Sues F.D.A. Over Right to Discuss Off-Label Uses”. New York Times. Retrieved 17 May 2017.
- Jump up^ Andrew Pollack for the New York Times. 7 August 2015 Court Forbids F.D.A. From Blocking Truthful Promotion of Drug
- Jump up^ Katie Thomas for the New York Times. 8 March 2016 F.D.A. Deal Allows Amarin to Promote Drug for Off-Label Use
 |
|
| Names | |
|---|---|
| IUPAC name
Ethyl (5Z,8Z,11Z,14Z,17Z)-eicosa-5,8,11,14,17-pentaenoate
|
|
| Other names
Eicosapentaenoic acid ethyl ester; Ethyl eicosapentaenoate; Eicosapent; Icosapent ethyl; EPA ethyl ester; E-EPA
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
|
PubChem CID
|
|
| Properties | |
| C22H34O2 | |
| Molar mass | 330.51 g·mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
////////////Icosapent ethyl, fda 2012, Timnodonic acid ethyl ester, Vascepa, AMR 101, AMR-101, E-EPA, Ethyl eicosapentaenoic acid , Fast-track status, Orphan drug designation
CCOC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CC


